Type 2 diabetes – advances in investigation and management

Major advances range from improved strategies for the detection of type 2 diabetes and screening for established and emerging complications to pharmacotherapy that offers cardiovascular and kidney protection.
- Type 2 diabetes is increasing in prevalence and is associated with a high risk of developing cardiovascular and kidney diseases.
- Measurement of glycated haemoglobin remains the gold standard for assessing glycaemic control, with a general target of less than 7% (53 mmol/mol), although this should be individualised to the person with type 2 diabetes.
- Type 2 diabetes is increasingly associated with complications such as heart failure, cognitive impairment and depression, malignancy and fatty liver disease.
- A multifactorial approach targeting glycaemic, blood pressure, lipid and weight control, as well as modifying lifestyle factors, are required for the optimal management of type 2 diabetes.
- Sodium-glucose cotransporter-2 (SGLT-2) inhibitors should be used in people with type 2 diabetes who have established cardiovascular disease (or who are at high risk of cardiovascular disease), heart failure or chronic kidney disease (CKD).
- Finerenone, which was PBS listed in July 2023, offers renoprotection and should be used in conjunction with SGLT-2 inhibitors in people with type 2 diabetes and CKD.
- Tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide (GLP-1) receptor agonist, became available in Australia in September 2023 as an agent to further manage type 2 diabetes and its associated metabolic abnormalities.
- Type 2 diabetes remission can be achieved with significant weight loss in people who are overweight or obese, including through lifestyle changes or bariatric surgery.
The prevalence of type 2 diabetes is increasing. The number of people living with diabetes in Australia increased almost 2.8-fold between 2000 and 2020, from 460,000 to 1.3 million people.1 People with type 2 diabetes are at increased risk of diabetes-associated complications that include cardiovascular and kidney disease. Although there is evidence that the rate of complications is declining, disease burden remains high because of increasing diabetes prevalence and greater longevity. Fortunately, there have been major advances in the management of type 2 diabetes and diabetes-associated complications. This article outlines recent advances in the investigation and management of type 2 diabetes, with a focus on cardio-kidney health.
Investigations
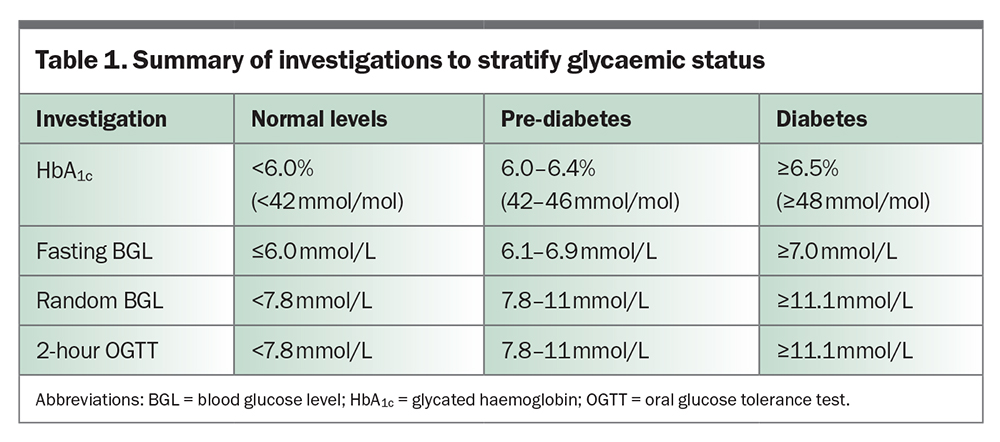
Measurement of glycated haemoglobin (HbA1c) and blood glucose levels (BGLs) is key to diagnosing type 2 diabetes and assessing glycaemic control. In asymptomatic patients, repeated testing with the following results is diagnostic of diabetes (Table 1):
- HbA1c 6.5% (48 mmol/mol) or more
- fasting BGL 7 mmol/L or more
- random or two-hour oral glucose tolerance test BGL 11.1 mmol/L or more.
For patients with type 2 diabetes, general management targets are:
- HbA1c 7% (53 mmol/mol) or less
- fasting/preprandial BGL 4.0 to 7.0 mmol/L
- postprandial BGL 5.0 to 10.0 mmol/L.
However, individualisation is required for some patients. This could include tighter targets for people with a shorter duration of type 2 diabetes taking metformin only, or less stringent targets for people with a long duration of diabetes and high burden of complications who are taking insulin or have recurrent hypoglycaemia.
Complications screening
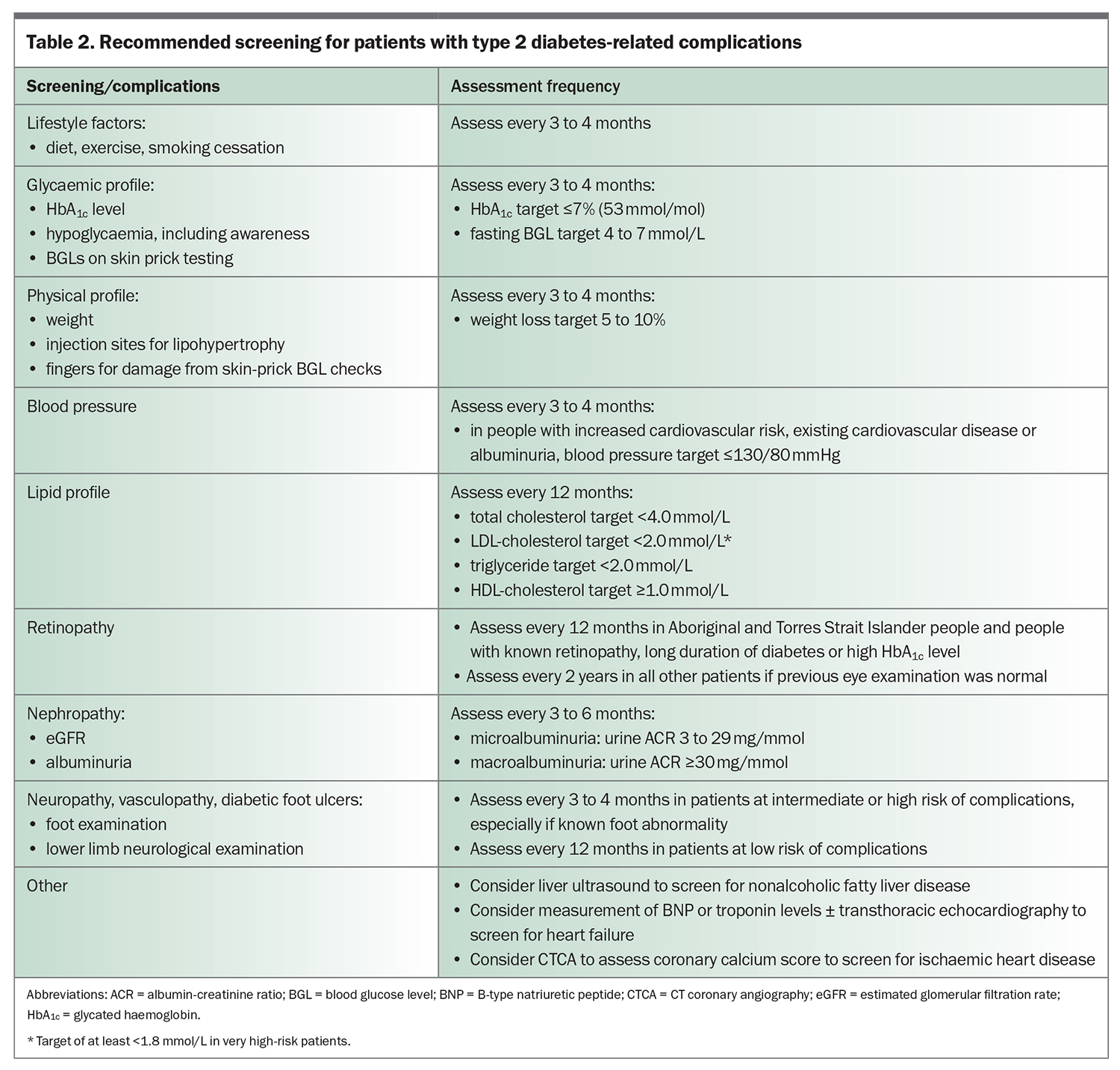
It is imperative that patients with type 2 diabetes undergo regular screening for diabetes-associated complications (Table 2). Microvascular complications of type 2 diabetes include nephropathy, retinopathy and neuropathy. Macrovascular complications include stroke, myocardial infarction and peripheral vascular disease. Regular monitoring of glycaemia, blood pressure, lipid profiles, kidney function and urine albumin-creatinine ratio (ACR), as well as eye checks and feet examination, play a central role in preventing the development and progression of diabetes-related complications.
Routine screening for coronary artery disease is not recommended in asymptomatic people, although a low threshold for investigation is recommended for patients with atypical cardiac symptoms or resting ECG abnormalities. Coronary calcium score or CT coronary angiography can be used to assess the risk of future cardiovascular events in people with type 2 diabetes. Heart failure is increasingly recognised as a complication of type 2 diabetes. Routine screening with echocardiography in asymptomatic people is not considered cost effective and therefore is not used in clinical practice. The American Diabetes Association consensus guidelines promote measurement of B-type natriuretic peptide or high-sensitivity cardiac troponin levels in symptomatic people with diabetes to help identify those at high risk of heart failure who need to proceed to echocardiography.2
Type 2 diabetes is associated with various other complications. Intracerebral microvascular damage from type 2 diabetes has been linked with increased risk of ischaemic and haemorrhagic stroke, cognitive dysfunction and depression.3 Type 2 diabetes and obesity increase the risk of cancer, particularly endometrial, liver, breast, pancreatic and colorectal cancers,4 although cancer screening guidelines currently remain the same in people with or without diabetes. Type 2 diabetes is also associated with nonalcoholic fatty liver disease, progression to nonalcoholic steatohepatitis and advanced fibrosis, and hepatocellular carcinoma. International guidelines suggest screening for nonalcoholic fatty liver disease with liver ultrasound, irrespective of liver enzyme levels, in patients with type 2 diabetes.5
Glycaemic management
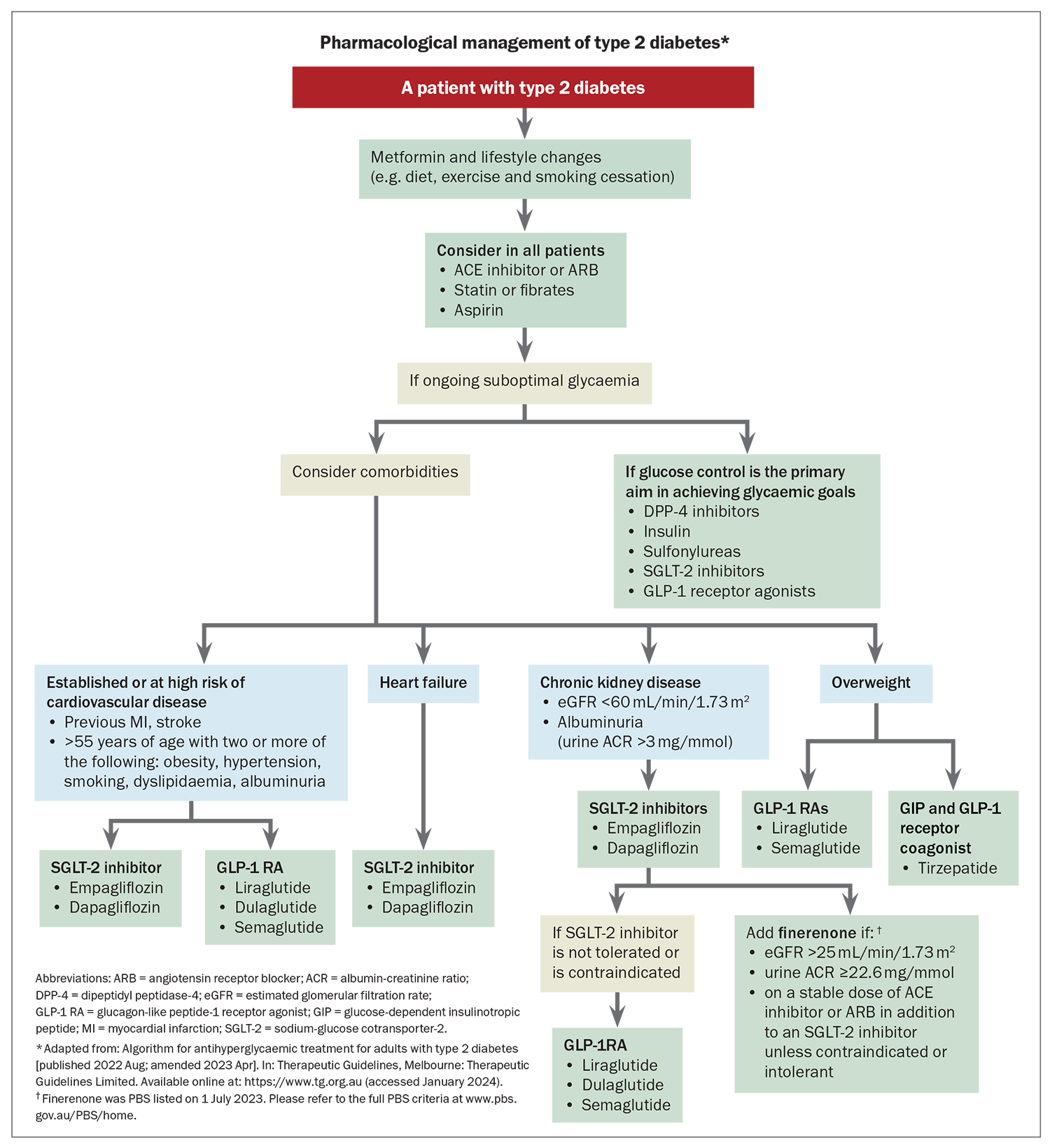
A summary of the action, potential benefits and place in management of the major classes of glucose-lowering medications is presented in the Flowchart. Please also refer to the Australian Type 2 Diabetes Glycaemic Management Algorithm (current update May 2023, see: www.diabetessociety.com.au/guideline/australian-type-2-diabetes-glycaemic-management-algorithm-august-2022/).
Metformin
Metformin, together with lifestyle changes, is the backbone of management of type 2 diabetes and is typically continued lifelong. Metformin reduces HbA1c levels by 0.5 to 1.0% (5.5 to 10 mmol/mol) and is weight neutral. The landmark United Kingdom Prospective Diabetes Study and its 10-year follow-up study showed that metformin was associated with reduced cardiovascular and all-cause mortality.6,7 Metformin may also reduce the incidence of cancer and dementia, and is beneficial in patients with polycystic ovary syndrome, fatty liver disease and inflammatory bowel disease.8 Side effects associated with metformin use include gastrointestinal upset and, rarely, lactic acidosis. Metformin is generally not recommended if the estimated glomerular filtration rate (eGFR) is less than 30 mL/min/1.73 m².9
SGLT-2 inhibitors
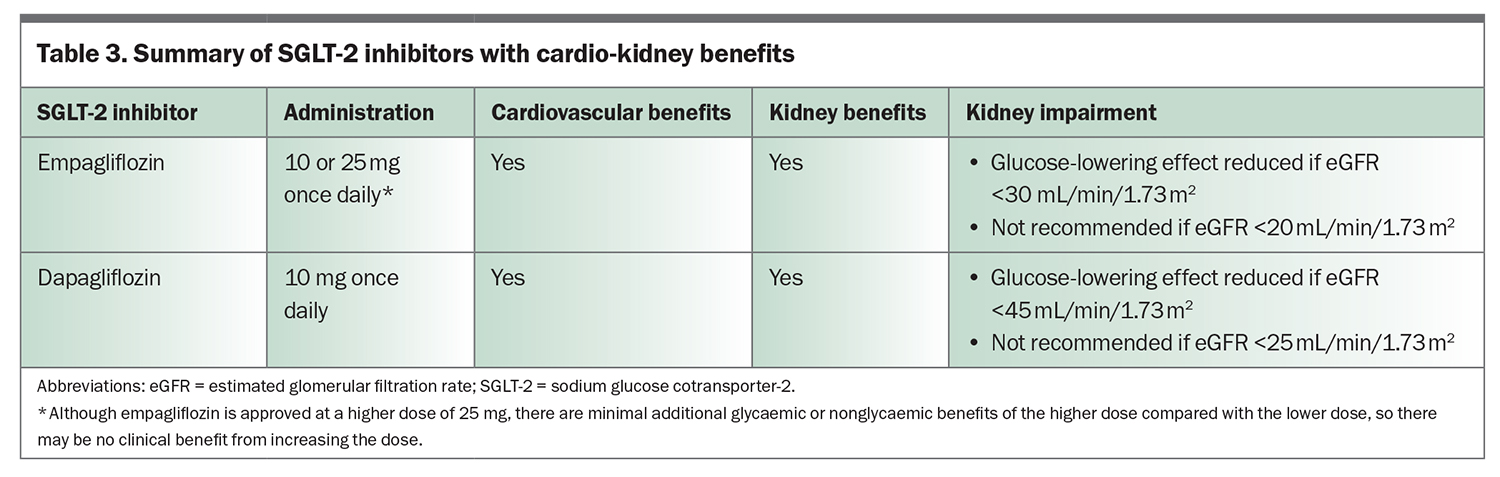
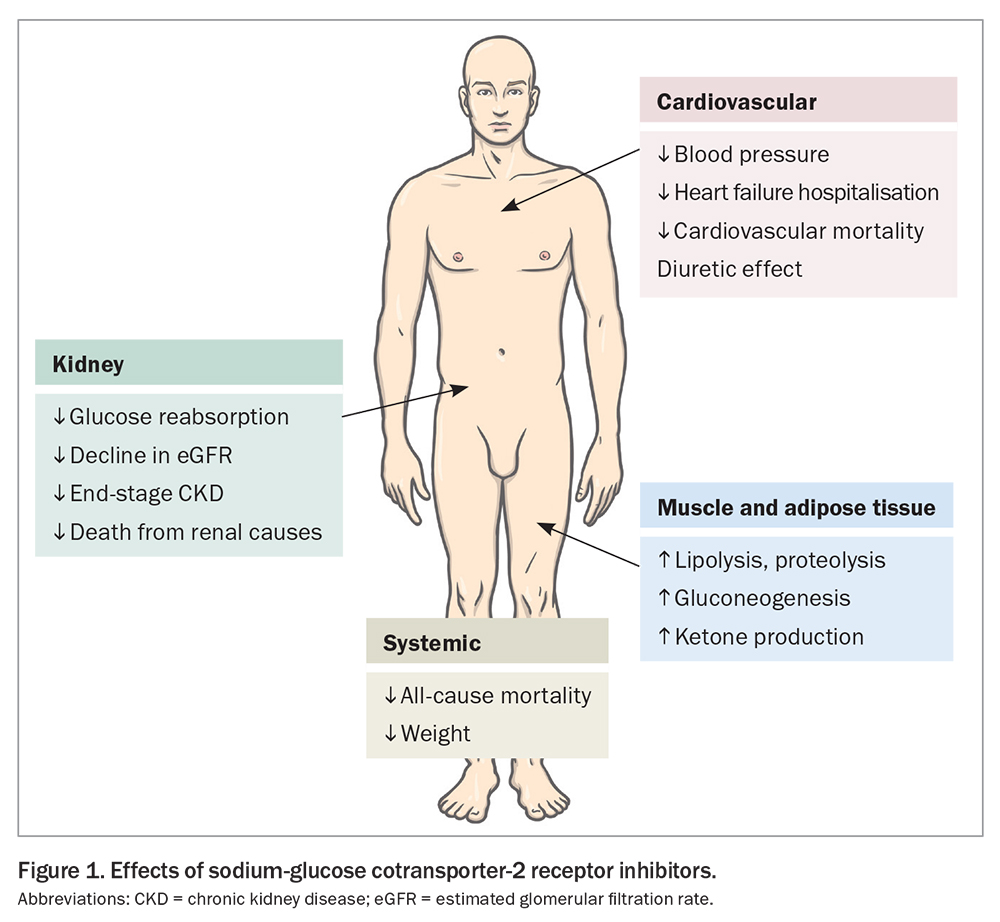
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors, empagliflozin and dapagliflozin, have gained popularity owing to multiple trials demonstrating their cardio-kidney benefits (Table 3). SGLT-2 inhibitors increase urinary glucose excretion (Figure 1), with an HbA1c reduction of 0.4 to 0.8% (4.5 to 9 mmol/mol) when used with metformin. Significantly greater HbA1c reductions are seen when baseline glycaemic control is poor. SGLT-2 inhibitors reduce weight and blood pressure and confer a low risk of hypoglycaemia. Adverse effects include polyuria, thirst, fungal genitourinary infections and euglycaemic ketoacidosis.
SGLT-2 inhibitors improve cardiovascular outcomes, particularly in people with cardiovascular disease (or at high risk of cardiovascular disease) or heart failure. Use of SGLT-2 inhibitors in patients with type 2 diabetes who have cardiovascular disease or who are at high risk of cardiovascular events has been shown to reduce cardiovascular and all-cause mortality and heart failure hospitalisation.10 In people with heart failure with either reduced ejection fraction (left ventricular ejection fraction [LVEF] <40%) or preserved or mildly reduced ejection fraction (LVEF ≥40%), SGLT-2 inhibitors have also been shown to reduce heart failure exacerbations, hospitalisation and cardiovascular death.11,12
Use of SGLT-2 inhibitors improves kidney outcomes, particularly in people with kidney impairment and albuminuria (macroalbuminuria: urine ACR ≥30 mg/mmol). In patients with chronic kidney disease (CKD), the risk of progression of kidney disease, end-stage kidney failure or death from renal or cardiovascular causes were significantly lower with use of SGLT-2 inhibitors than with placebo.13-15
The cardio-kidney protection achieved with use of SGLT-2 inhibitors is likely to be independent of glycaemia. Mediation analyses within outcome trials have demonstrated that the results were independent of the magnitude of HbA1c decline, and similar effects are also seen in people without diabetes. Current international consensus guidelines suggest that patients with type 2 diabetes and cardiovascular disease (or who are at high risk of cardiovascular disease), heart failure or CKD (eGFR <60 mL/min/1.73 m²) or albuminuria (urine ACR ≥3 mg/mmol) should receive an SGLT-2 inhibitor, in addition to metformin, irrespective of baseline HbA1c levels.16 Dapagliflozin is PBS listed for a diagnosis of CKD in patients with an eGFR 25 to 75 mL/min/1.73 m² and urine ACR 22.6 to 565 mg/mmol that has been stable with use of an ACE inhibitor or angiotensin receptor blocker (ARB).17 The glucose-lowering effect of SGLT-2 inhibitors can be reduced with kidney impairment; therefore, additional glucose-lowering therapies may be required, although the cardio-kidney protective effects are maintained.
GLP-1 receptor agonists
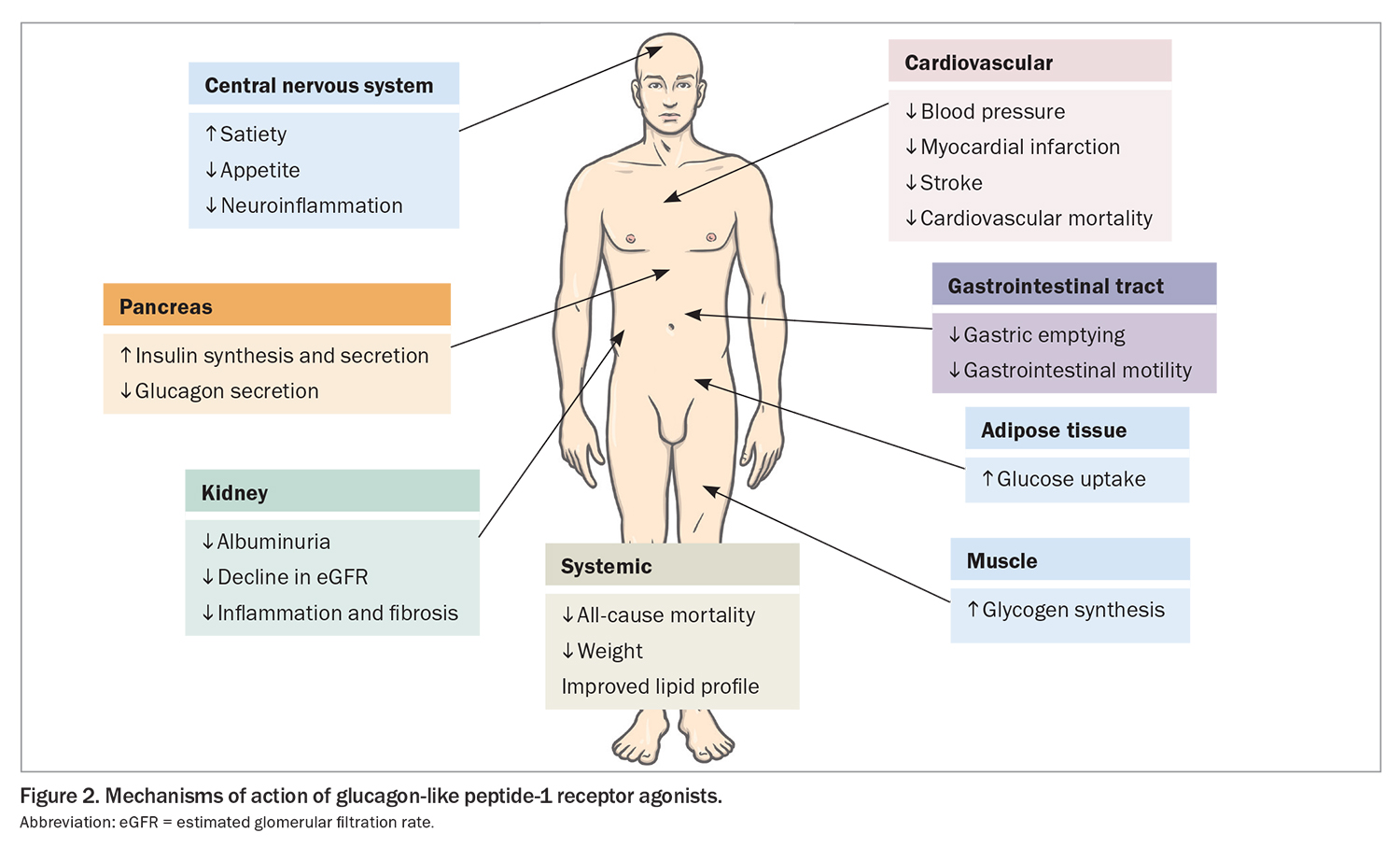
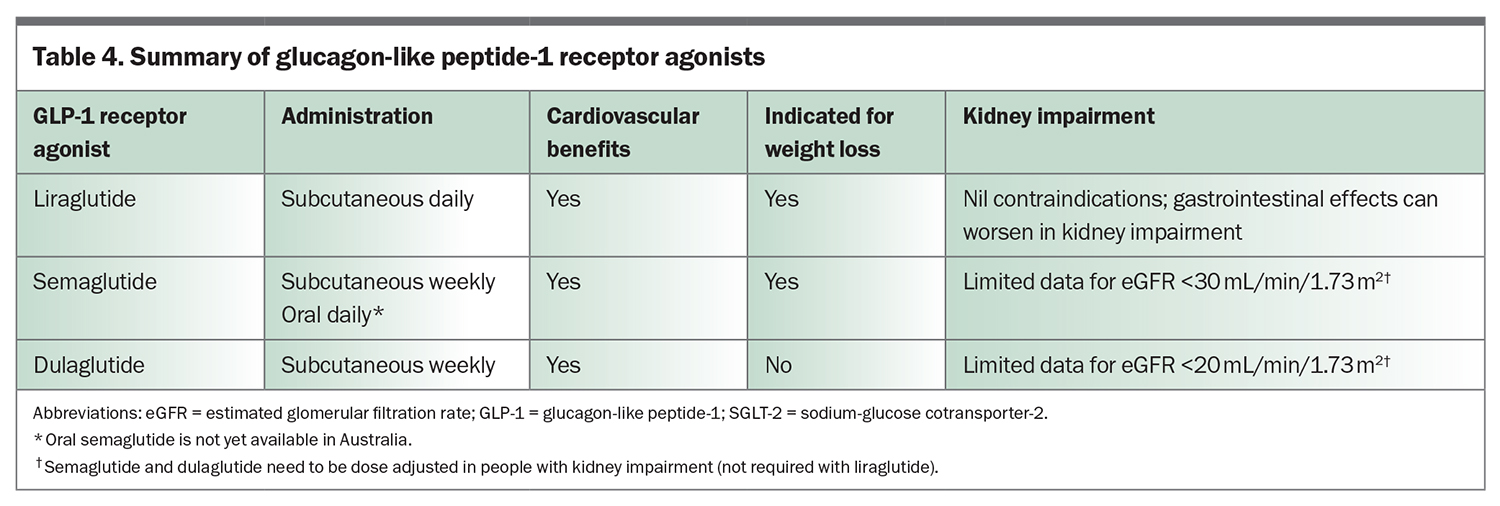
Glucagon-like peptide-1 (GLP-1) receptor agonists, including semaglutide, liraglutide and dulaglutide, stimulate glucose-dependent insulin secretion from the pancreas, but also slow gastric emptying to minimise postprandial hyperglycaemia and promote satiety via centrally mediated effects (Figure 2). GLP-1 receptor agonists reduce HbA1c by 0.6 to 1.5% (6.5 to 16.5 mmol/mol) when used with metformin and are administered subcutaneously (oral semaglutide is not yet available in Australia). GLP-1 receptor agonists also reduce weight and blood pressure and have a modest effect on improving lipid profiles. They can cause gastrointestinal upset, but this generally dissipates with continued treatment.
GLP-1 receptor agonists offer cardiovascular benefit (Table 4). Multiple cardiovascular outcomes trials involving people with cardiovascular disease or at high risk of cardiovascular disease have variably reported a reduced incidence of myocardial infarction, stroke and cardiovascular and all-cause mortality with use of GLP-1 receptor agonists.18-21 However, unlike SGLT-2 inhibitors, GLP-1 receptor agonists, to date, have not been shown to be beneficial in preventing heart failure exacerbations or hospitalisation for heart failure.
Cardiovascular outcomes trials in patients with relatively preserved kidney function suggest that GLP-1 receptor agonist use reduces macroalbuminuria and potentially eGFR decline, with benefits being most pronounced in participants with pre-existing CKD or albuminuria. To date, no trial has shown that GLP-1 receptor agonists slow progression to end-stage CKD. The Effect of Semaglutide Versus Placebo on the Progression of Renal Impairment in Subjects With Type 2 Diabetes and Chronic Kidney Disease (FLOW) trial is the first specific kidney outcome trial involving GLP-1 receptor agonists and is expected to finish in 2024.
GLP-1 receptor agonists cause weight loss. In the Effect and Safety of Semaglutide 2.4 mg Once-Weekly in Subjects with Overweight or Obesity (STEP 1) trial, involving people with obesity or overweight (body mass index [BMI] >30 kg/m2 or >27 kg/m2 in people with weight-related conditions), high-dose semaglutide (2.4 mg per week) in people without diabetes resulted in 12.4% more weight reduction compared with placebo.22 In contrast, the STEP-2 trial showed that high-dose semaglutide resulted in only 6.2% more weight loss compared with placebo in adults with diabetes and BMI of 27 kg/m2 or more, and HbA1c level 7 to 10% (53 to 86 mmol/mol).23 This trial also showed that high-dose semaglutide use was associated with an absolute drop in HbA1c of 1.2% compared with placebo. Furthermore, although high-dose semaglutide resulted in 2.7% greater weight loss than semaglutide 1.0 mg per week, HbA1c differences between the two doses of semaglutide were of marginal significance (absolute difference 0.2%).
Current guidelines suggest that an SGLT-2 inhibitor or GLP-1 receptor agonist should be used in people with type 2 diabetes and cardiovascular disease or in those at high risk of cardiovascular disease, regardless of HbA1c levels. Of note, the current PBS listings for semaglutide and dulaglutide require a patient with type 2 diabetes to have an HbA1c measurement greater than 7% before initiation of a GLP-1 receptor agonist. It is expected that further PBS restrictions for prescribing GLP-1 receptor agonists will be introduced in the near future. A GLP-1 receptor agonist should be used if SGLT-2 inhibitors are contraindicated or not tolerated.16 Unfortunately, there is currently a global shortage of dulaglutide and semaglutide, which is not expected to improve until late 2024. Intensification of lifestyle measures and alternative medications, such as liraglutide, SGLT-2 inhibitors, DPP-4 inhibitors or even short-term insulin therapy, should be used in the interim.
Tirzepatide
Tirzepatide is a glucose-dependent insulinotropic peptide and GLP-1 receptor coagonist that was recently TGA approved to treat hyperglycaemia in people with type 2 diabetes and became available in Australia in September 2023. Tirzepatide is administered subcutaneously as a once-weekly injection. Studies have reported HbA1c reductions of 1.6 to 2.6% (20 to 28 mmol/mol).24-28
Tirzepatide is associated with low risk of hypoglycaemia, improved lipid profile and reduced blood pressure. Tirzepatide is also associated with significant weight loss, with a study finding a mean weight reduction of 26% over 88 weeks when it was used in conjunction with diet and exercise.29 Another study found the weight loss effects of tirzepatide were greater than those of semaglutide.25 Tirzepatide has been approved for long-term weight management by the US Food and Drug Administration but not the TGA. Side effects include gastrointestinal upset, especially nausea.
Tirzepatide is potentially associated with cardio-kidney benefits. Trials to date have demonstrated cardiovascular safety.26-28 It is expected that further trials involving tirzepatide will also show cardiovascular protective effects and, possibly, favourable outcomes related to kidney health. A recent study showed that tirzepatide reduced progression of albuminuria and kidney function decline (especially in participants with eGFR <60 mL/min/1.73 m2).30 Trials are underway to definitively demonstrate the cardiovascular and kidney benefits of this medication. Unfortunately, there is currently a global shortage of tirzepatide that is not expected to improve until late 2024.
Other glucose-lowering therapies
Dipeptidyl peptidase-4 (DPP-4) inhibitors, including alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin, prevent the enzymatic breakdown of endogenous GLP-1, producing a HbA1c reduction of 0.5 to 0.6% (5.5 to 6.5 mmol/mol) when used with metformin. DPP-4 inhibitors are weight neutral with minimal risk of hypoglycaemia; however, they do not offer any specific cardio-kidney benefits. In fact, one trial found an increased risk of hospitalisation for heart failure with saxagliptin use.31 Some trials have suggested a reduction in albuminuria,but the independence of this effect from glucose lowering has yet to be established.32 Linagliptin is the only DPP-4 inhibitor that does not require dose reduction at an eGFR of less than 50 mL/min/1.73 m².
Sulfonylureas including glimepiride and modified-release gliclazide promote insulin secretion, offering an HbA1c reduction of 0.5 to 0.7% (5.5 to 7.5 mmol/mol) when used with metformin. Although effective for glucose lowering, sulfonylureas can cause hypoglycaemia (especially in people with kidney impairment) and weight gain. Studies have shown cardiovascular safety for glimepiride and modified-release gliclazide, but these medications do not offer any additional cardio-kidney benefits independent of glucose lowering.
Pioglitazone is the only thiazolidinedione still approved in Australia. Its use is limited by concerns regarding precipitation of heart failure, and increased risk of postmenopausal fractures, bladder cancer and macular oedema. Pioglitazone sensitises the effects of insulin, so it can be used in people with severe insulin resistance; however, other medications are now generally favoured. Rosiglitazone was withdrawn in 2010 following concerns regarding increased cardiovascular risk.
Acarbose inhibits alpha-glucosidase to delay carbohydrate digestion and glucose absorption to reduce postprandial glucose peaks, but it must be taken with the first few mouthfuls of food to be effective. Acarbose has been shown to reduce cardiovascular events, especially myocardial infarction and hypertension in people with impaired glucose tolerance,although these effects are not as pronounced as with metformin in people with type 2 diabetes.33 ,34 The use of acarbose may be limited by gastrointestinal adverse effects, including flatulence, bloating and diarrhoea.
Insulin therapy
Subcutaneous insulin is used to supplement endogenous insulin production and has a dose-dependent glucose-lowering effect. Many people with type 2 diabetes will eventually require insulin due to progressive insulin resistance and pancreatic beta-cell failure. However, insulin can cause weight gain and hypoglycaemia, and its efficacy is dependent on patient education and engagement. When insulin is started in patients with type 2 diabetes, an initial dose of 0.1 to 0.2 units/kg of long- or intermediate-acting insulin (alone or combined with short-acting insulin) is recommended. A timely insulin dose titration schedule is vital for all patients starting insulin therapy. If hyperglycaemia continues, short-acting insulin can be added, or the regimen changed to twice-daily mixed insulin.
Nonglucose-lowering adjunctive therapies
Finerenone
Finerenone is a nonsteroidal mineralocorticoid receptor antagonist that has no glucose-lowering effects but offers kidney benefits. Finerenone was recently approved by the TGA to delay the progressive decline of kidney function in adults with type 2 diabetes-associated CKD with albuminuria. In people with type 2 diabetes and albuminuric CKD, finerenone has been shown to significantly reduce the risk of kidney failure (kidney death or eGFR decrease of 57% or more). Finerenone also demonstrated cardiovascular safety, reduction in heart failure hospitalisation and, potentially, cardiovascular death and myocardial infarction.35 Finerenone does not cause off-target effects such as gynaecomastia. However, it can cause hyperkalaemia, although at lower rates than the steroidal mineralocorticoid receptor agonists spironolactone and eplerenone, so potassium levels should be monitored. Finerenone offers only very modest blood pressure-lowering effects compared with spironolactone or eplerenone.
Finerenone is TGA approved to delay progressive decline of kidney function and to reduce the risk of CV mortality and morbidity in adults with CKD (with albuminuria) associated with type 2 diabetes, in addition to standard of care. It is PBS listed for patients who fulfil the following criteria:
- eGFR of 25 mL/min/1.73 m2 or greater
- ACR of 22.6 mg/mmol or greater
- on a stable dose of either an ACE inhibitor or ARB
- do not have established heart failure with reduced ejection fraction
- on an SGLT-2 inhibitor unless medically contraindicated or intolerant (refer to the full PBS clinical criteria at www.pbs.gov.au/pbs/home).
Other multifactorial interventions
People with type 2 diabetes have a two to six times increased risk of cardiovascular death compared with people without diabetes. Multifactorial, target-driven interventions remain the cornerstone of primary and secondary cardiovascular protection in people with diabetes. This includes blood pressure and hyperlipidaemia management with the use of ACE inhibitors or ARBs and statins, respectively, in addition to glycaemic management and attention to lifestyle factors. The Intensified Multifactorial Intervention in Patients With Type 2 Diabetes and Microalbuminuria (Steno-2) study showed that intensive, multifactorial risk-factor modification in people with type 2 diabetes, hypertension and microalbuminuria resulted in significantly reduced microvascular complications (except peripheral neuropathy), cardio vascular events, heart failure hospitalisation, progression to end-stage CKD and all-cause mortality, with all outcomes being reduced by more than 50%.36 A contemporary approach would also involve prescribing cardiovascular protective medications, such as SGLT-2 inhibitors or GLP-1 receptor agonists, for people with type 2 diabetes.
Current guidelines recommend multifactorial risk-factor modification in people with type 2 diabetes aged over 60 years with microalbuminuria or moderate-severe CKD (eGFR <45 mL/min/1.73 m2). Lifestyle interventions, including smoking cessation, exercise and weight control, are paramount in reducing the risk of development and progression of type 2 diabetes-associated complications. Increased physical activity is an essential component of lifestyle advice for people with diabetes. Glycaemic targets for people with type 2 diabetes include HbA1c less than 7% (53 mmol/mol), with individualisation if required. Blood pressure targets are 140/90 mmHg or less in general, or 130/80 mmHg or less in people with albuminuria or at high cardiovascular risk, although the American Diabetes Association has recently lowered its general blood pressure target to less than 130/80 mmHg. General lipid targets include total cholesterol level of less than 4.0 mmol/L, HDL level of 1 mmol/L or more, LDL level of less than 2.0 mmol/L (and at least <1.8 mmol/L in people with cardiovascular disease), and triglyceride levels of less than 2.0 mmol/L. Statins may be indicated for people with cardiovascular disease or those at high cardiovascular risk regardless of lipid profile. Urine ACR should be less than 3.5 mg/mmol in women and less than 2.5 mg/mmol in men.
Weight loss and type 2 diabetes remission
Type 2 diabetes remission can be achieved with substantial weight reduction from dietary changes or bariatric surgery in people who are overweight or obese, and is more likely within the first few years of diagnosis. Type 2 diabetes remission is defined as an HbA1c level less than 6.5% (48 mmol/mol) sustained for three months without using glucose-lowering medication. Dietary changes include very low energy diets of 3300 kJ/day or less, or ketogenic diets high in fat and protein and low in carbohydrates. Metabolic surgery includes gastric band, gastric sleeve or gastric bypass, and resulted in 23 kg more weight loss and 1.3% (15 mmol/mol) greater reduction in HbA1c compared with GLP-1 receptor agonists in a meta-analysis.37
Metabolic surgery should be considered for people with type 2 diabetes and a BMI of 30 kg/m² or more, in whom diet, exercise and pharmacotherapy have been unsuccessful.38 In one study, metabolic surgery resulted in type 2 diabetes remission in 37.5% of participants, which was sustained over the 10-year follow-up period. People who relapsed after metabolic surgery maintained better glycaemia (mean HbA1c level 6.7% [50 mmol/mol]) compared with medical therapy. Metabolic surgery also significantly reduced the incidence of diabetes- related complications compared with medical therapy (5.0% vs 72.2%),39 a finding also seen in other observational studies. However, until more evidence becomes available, patients in remission should continue to receive regular screening for diabetes complications.
Conclusion
Type 2 diabetes is a prevalent condition associated with many established and emerging complications. Strategies for detecting and managing type 2 diabetes and associated complications continue to improve. The use of HbA1c to stratify glycaemic status and hence diagnose diabetes is now routine in clinical practice. Regular monitoring of HbA1c supplemented by BGL measurements remain the gold standard for assessing glycaemia in people with type 2 diabetes. Routine screening for coronary artery disease is still not recommended, although assessing the coronary calcium score can be a useful cardiovascular risk stratification approach. Heart failure is an emerging complication of type 2 diabetes, with some international guidelines recommending annual screening.
Over the past decade there have been major advances in the pharmacological management of type 2 diabetes, with the introduction of medications that improve glycaemia (with a low risk of hypoglycaemia), promote weight loss and provide cardio-kidney protection. The wider availability of tirzepatide as an agent to further manage type 2 diabetes and its associated metabolic abnormalities and complications is eagerly expected. In addition, attention to lifestyle factors and use of a multifactorial, target-driven approach to the management of glycaemia, blood pressure and lipid control remain cornerstones of type 2 diabetes management. ET
COMPETING INTERESTS: Dr Lin: None. Professor MacIsaac was a recipient of St Vincent’s Hospital Research Endowment Fund 2021–2023 and the Australian Government’s Medical Research Future Fund (established by MTPConnect’s Targeted Transition Research Accelerator initiative); and has received study funding from Medtronic Pty Ltd, GlySens Pty Ltd and Insulet Pty Ltd. He has been on Advisory Boards for Astra Zeneca (2021, 2023), Novo Nordisk (2021), Boehingher Ingelheim (2022), Bayer (2021–2022) and Servier (2021); and received speaker’s fees from Boehingher Ingelheim (2021, 2023), Novo Nordisk (2021–2022), Eli Lilly (2022) and Servier (2021). Professor Ekinci has received research funding for EIE’s institution from Novo Nordisk, Eli Lilly, Sanofi, Boehringer, Versanis and Endogenex; consulting fees from Eli Lilly, Boehringer and Bayer (funds received are donated to The University of Melbourne for diabetes research); and payment honoraria from Eli Lilly, Boehringer and Amgen (funds received are donated to The University of Melbourne for diabetes research); and support to attend a national diabetes meetings from Eli Lilly.
References
1. Australian Institute of Health and Welfare. Diabetes: Australian facts 2020. Available online at: https://www.aihw.gov.au/reports/diabetes/diabetes/contents/how-common-is-diabetes/all-diabetes (accessed July 2023).
2. Pop-Busui R, Januzzi JL, Bruemmer D, et al. heart failure: an underappreciated complication of diabetes. a consensus report of the American Diabetes Association. Diabetes Care 2022; 45: 1670-1690.
3. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol 2020; 8: 325-336.
4. Bonagiri PR, Shubrook JH. Review of associations between type 2 diabetes and cancer. Clin Diabetes 2020; 38: 256-265.
5. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388-1402.
6. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 1999; 48: 643-648.
7. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577-1589.
8. Top WMC, Kooy A, Stehouwer CDA. Metformin: a narrative review of its potential benefits for cardiovascular disease, cancer and dementia. Pharmaceuticals 2022; 15: 04.
9. Tanner C, Wang G, Liu N, Andrikopoulos S, Zajac JD, Ekinci EI. Metformin: time to review its role and safety in chronic kidney disease. Med J Aust 2019; 211: 37-42.
10. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117-2128.
11. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995-2008.
12. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387: 1089-1098.
13. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295-2306.
14. Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
15. Herrington W, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2022; 388: 117-127.
16. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022; 45: 2753-2786.
17. NPS Medicine Wise. Dapagliflozin for chronic kidney disease 2022. Available online at: https://www.nps.org.au/radar/articles/dapagliflozin-for-chronic-kidney-disease (accessed July 2023).
18. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
20. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardio vas-cular outcomes in patients with type 2 diabetes. N Engl J Med 2019; 381: 841-851.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo.
22. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989-1002.
23. Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021; 397: 971-984.
24. Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021; 398: 143-155.
25. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
26. Ludvik B, Giorgino F, Jodar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 2021; 398: 583-598.
27. Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021; 398: 1811-1824.
28. Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 2022; 327: 534-545.
29. Aronne LJ, Sattar N, Horn DB, et al; SURMOUNT-4 Investigators. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA 2024; 331: 38-48.
30. Heerspink HJL, Sattar N, Pavo I, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol 2022; 10: 774-785.
31. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317-1326.
32. Perkovic V, Toto R, Cooper ME, et al. Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the CARMELINA Randomized Trial. Diabetes Care 2020; 43: 1803-1812.
33. Chiasson J-L, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance The STOP-NIDDM Trial. JAMA 2003; 290: 486-494.
34. Chang C-H, Chang Y-C, Lin J-W, Chen S-T, Chuang L-M, Lai M-S. Cardiovascular risk associated with acarbose versus metformin as the first-line treatment in patients with type 2 diabetes: a nationwide cohort study. J Clin Endocrinol Metab 2015; 100: 1121-1129.
35. Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2021; 43: 474-484.
36. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383-393.
37. Sarma S, Palcu P. Weight loss between glucagon-like peptide-1 receptor agonists and bariatric surgery in adults with obesity: a systematic review and meta-analysis. Obesity 2022; 30: 2111-2121.
38. Australian Diabetes Society. The Australian obesity management algorithm 2016. Available online at: https://www.diabetessociety.com.au/guideline/obesity/ (accessed May 2023)
39. Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021; 397: 293-304.