Diabetic kidney disease: strategies for holistic management

Despite advances in treatment options, morbidity and mortality rates associated with diabetic kidney disease continue to rise. Early diagnosis and management significantly improve outcomes and slow progression to end-stage kidney disease. Criteria for diagnosis of diabetic kidney disease are a reduced estimated glomerular filtration rate or an elevated urinary albumin level in the setting of diabetes. Renin-angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors demonstrate substantial benefits in cardiovascular and renal protection and should be considered early as part of holistic management.
- Diabetic kidney disease (DKD) is a leading cause of chronic kidney disease (CKD) and accounts for a significant percentage of patients commencing dialysis. CKD mortality rates continue to rise and increased research and comprehensive management are required.
- Early detection and intervention in patients with DKD are crucial for improving outcomes and preventing progression to end-stage kidney disease.
- Effective management aims to reduce albuminuria and cardiovascular risk and slow the decline in kidney function.
- A multidisciplinary approach should include lifestyle modifications, glycaemic control, blood pressure management, lipid control and the use of targeted medications for renoprotection.
- Evidence-based pharmacotherapy for preserving kidney function in patients with diabetes involves a tiered approach, starting with an ACE inhibitor or angiotensin receptor blocker (ARB) as first-line therapy, followed by a sodium-glucose cotransporter-2 (SGLT-2) inhibitor as second-line therapy and a nonsteroidal mineralocorticoid antagonist as third-line therapy.
- Continued research and emerging treatments aimed at various pathways within diabetes and DKD hold promise for improved treatment options in the future.
Diabetic kidney disease (DKD) is the primary cause of chronic kidney disease (CKD) and the most common reason for new commencement of dialysis in Australia, accounting for 40% of dialysis initiations.1 Over the past two decades, the prevalence of diabetes has risen, as has the number of patients with end-stage kidney disease (ESKD), particularly in the Asia-Pacific region. Although mortality rates for other chronic illnesses such as ischaemic heart disease, stroke and chronic respiratory disease have declined, CKD mortality continues to rise.2 Regrettably, the investment in CKD research, education and screening falls short of addressing the immense impact and societal burden of the disease, and further action is required to mitigate its devastating effects and complications.
Improving outcomes for patients with DKD relies on implementing evidence-based care, often necessitating a multidisciplinary approach contingent on the stage of CKD. Before the development of treatment with renin-angiotensin system (RAS) inhibitors, the natural course of diabetic nephropathy in patients with type 1 diabetes typically involved albuminuria after the first decade followed by a declining glomerular filtration rate (GFR) in the subsequent decade, leading to ESKD in the third decade and beyond. Early trials investigating captopril demonstrated remarkable success, reducing progression to ESKD by 50%.3 Despite these early successes, a considerable number of patients have developed or continue on the path to ESKD over the past 30 years.1,2
In the past decade, clinical trials of novel pharmacotherapies have shown substantial benefits in cardiovascular (CV) health, kidney function and, in some instances, mortality for patients with diabetes, both with and without CKD. Hence, it is imperative that these evidence-based therapies are known, considered and appropriately prescribed to patients within these populations. This article outlines contemporary management of patients with CKD associated with type 1 or type 2 diabetes.
Pathophysiology
Early pathological manifestations of DKD include hyperfiltration (at the level of a single nephron) and kidney hypertrophy resulting from various physiological abnormalities. A key driving factor is the upregulation of sodium and glucose reabsorption in the proximal tubules.4,5 This initial hyperfiltration progresses to early structural changes in the kidney, such as thickening of the glomerular basement membrane and proximal tubule hypertrophy. Subsequent podocyte damage leads to the onset of microalbuminuria, which is a predictor of advancing kidney disease. Even as some nephrons become obsolete and the overall GFR decreases, the remaining nephrons undergo hyperfiltration and experience glomerular hypertension with ultimate progression to kidney disease.
Although this course is often seen in patients with type 1 diabetes progressing to DKD, the path to kidney failure in those with type 2 diabetes varies significantly. Notably, up to 40% of patients with type 1 diabetes (and a higher percentage of patients with type 2 diabetes) may not develop albuminuria, yet a significant portion of nonalbuminuric patients still progress to renal failure.6
Definition, diagnosis and screening for DKD
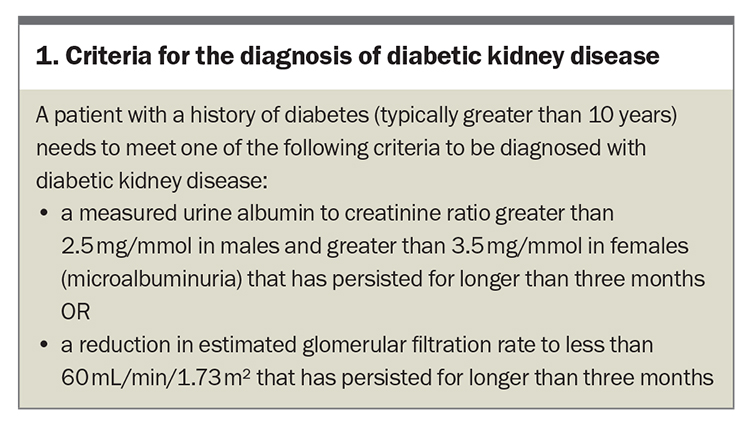
The clinical diagnosis of DKD is considered when the patient has a history of diabetes (typically for 10 years or more), as well as elevated urinary albumin levels with or without a reduced estimated glomerular filtration rate (eGFR) for three months or longer (Box 1). This is more relevant in patients with type 1 diabetes who have a clear onset of disease. However, additional clinical and laboratory features are required in patients with type 2 diabetes, where the disease may have been present before a formal diagnosis is achieved. Notably, diabetic retinopathy often precedes or occurs simultaneously with diabetic nephropathy, serving as a valuable clue to the presence of DKD.
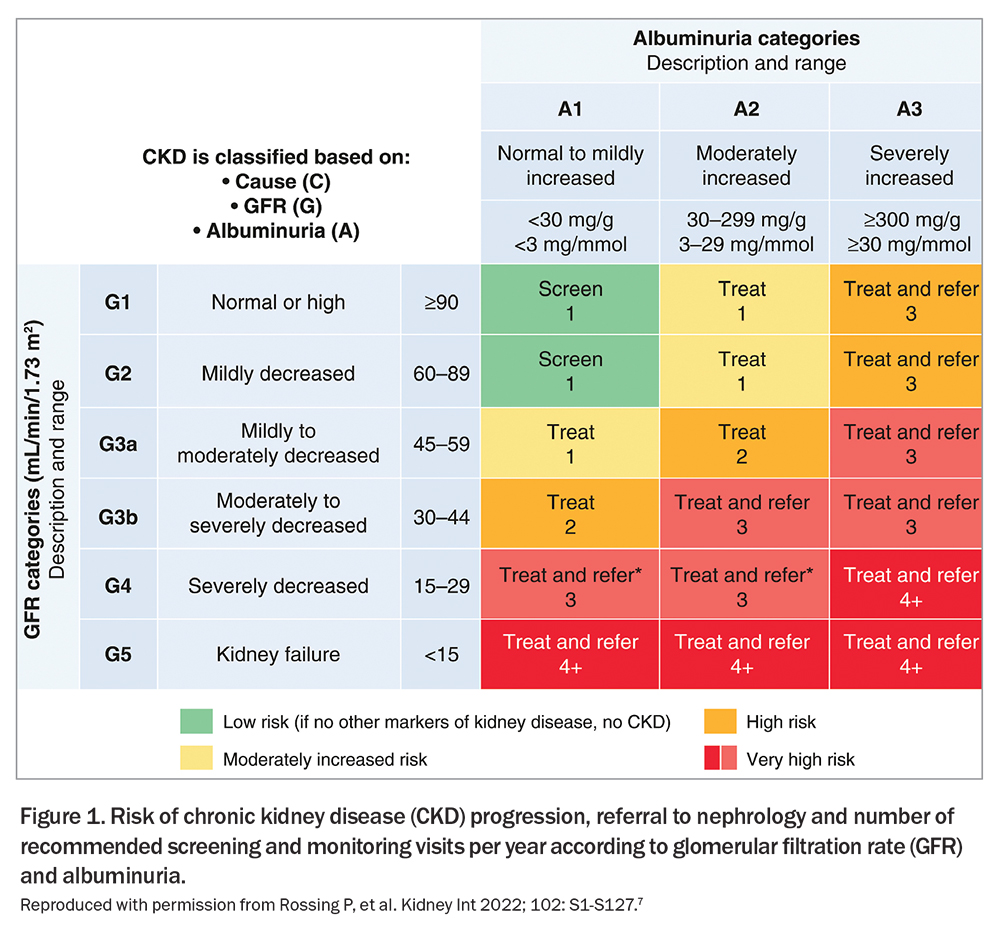
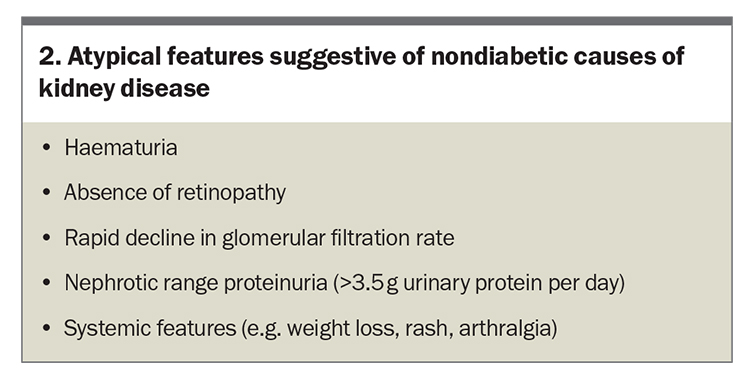
In the absence of complicating risk factors (e.g. urinary tract infection), haematuria is not a common indicator of DKD and its presence may suggest alternative causes of CKD. Early DKD typically manifests with preserved eGFR alongside varying levels of albuminuria, signifying the need for aggressive intervention to impede its progression (Figure 1).7 If atypical features of DKD are detected (Box 2), a kidney biopsy is recommended as over half of suspected DKD cases involve an alternative or contributing kidney condition.8 Screening for other diabetes-related complications is imperative in patients diagnosed with DKD as the prevalence of foot ulceration, retinopathy and neuropathy markedly increases (if not already present).9
Principles of management of DKD
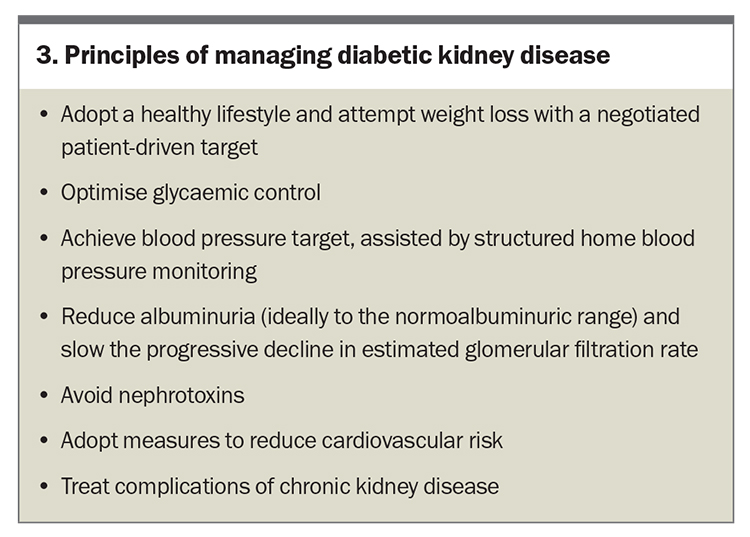
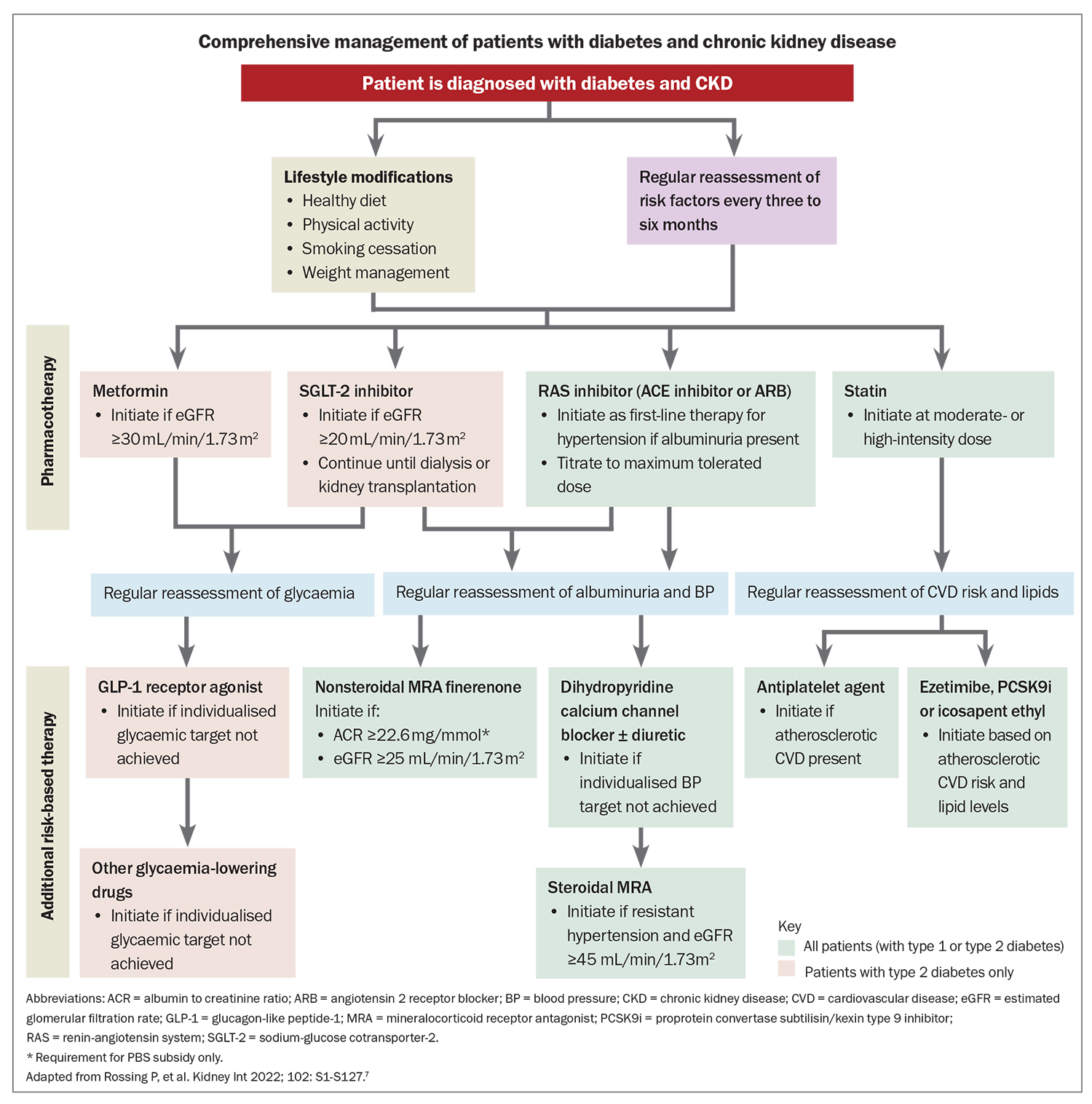
The primary objective of managing patients with DKD is to initiate treatment as early as possible to prevent or delay progressive kidney failure and mitigate associated CV risks (Box 3). These risks often curtail life expectancy before patients reach ESKD. Comprehensive management of patients with DKD is outlined in the Flowchart.7
Modify lifestyle factors
Management of diabetes relies on modification of lifestyle factors: diet, exercise, weight management and smoking cessation. The Primary Care-led Weight Management for Remission of Type 2 Diabetes (DiRECT) trial indicated that lifestyle changes and weight loss can trigger resolution of diabetes in up to 40% of patients in general practice.10
In patients with diabetes who smoke, behavioural modifications, nicotine replacement therapy and standard medications such as varenicline and bupropion can be prescribed. No dose adjustment is necessary with varenicline in patients with mild to moderate renal impairment. The recommended dose for patients with severe renal impairment is 1 mg daily (not twice daily). Prescription of varenicline is not recommended in patients with ESKD due to insufficient clinical experience. The effect of kidney disease on the pharmacokinetics of bupropion has not been studied; however, as its active metabolites are further metabolised and excreted by the kidneys, treatment should be initiated at reduced frequency or dosage in patients with renal impairment. Adverse effects to treatment (e.g. insomnia, dry mouth, seizures) are more likely to occur with reduced renal function. Smoking behaviour should be reviewed at every appointment due to the wide range of quit attempts, averaging between six and 30 per patient, for successful cessation.11
Optimise glycaemic control
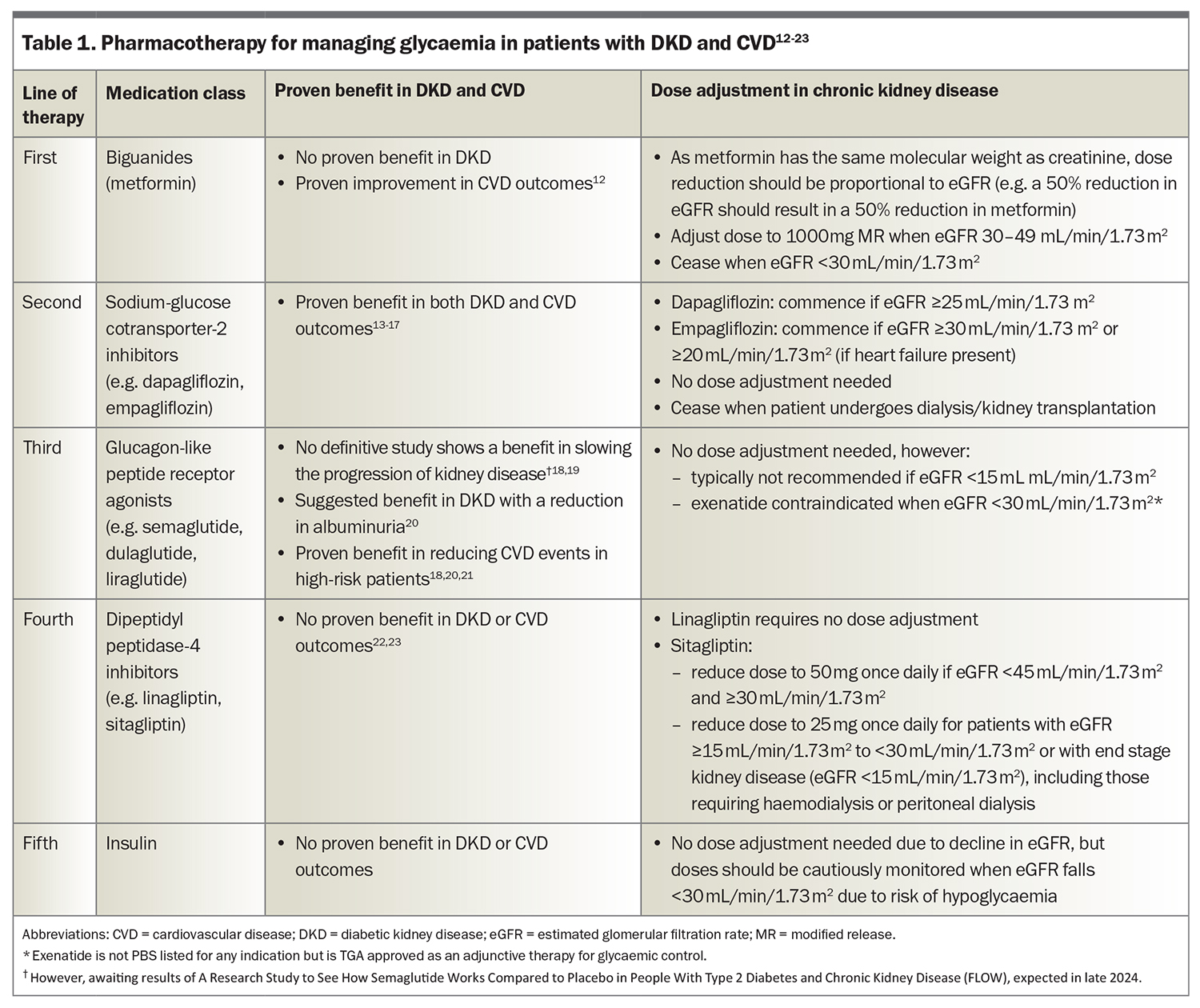
Glycaemic control should be personalised to suit individual patients (Table 1).12-23 The UK Prospective Diabetes Study demonstrated a 24% reduction in risk of diabetic nephropathy when patients maintained a mean glycated haemoglobin level of 2.8% (7.0 mmol/mol).24 However, subsequent studies have shown limited additional benefits from more intensive control beyond this threshold. A full review of agents for glycaemic control in patients with diabetes and DKD is beyond the scope of this article.
Manage blood pressure
Effective blood pressure management is essential for patients with DKD; however, optimal targets may differ among patients. A RAS inhibitor (ACE inhibitor or angiotensin receptor blocker [ARB]) is recommended as first-line therapy. Although studies in people without diabetes have favoured intensive control (blood pressure target <120 mmHg) for improved CV outcomes, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial did not demonstrate the same benefits in patients with diabetes. The ACCORD trial showed a marked reduction in stroke but it only included 401 patients with an eGFR of less than 60 mL/min/1.73 m2.25
The overall rate of decline in eGFR was higher with intensive blood pressure control in the Systolic Blood Pressure Intervention (SPRINT) (in both CKD and non-CKD cohorts), ACCORD and Study for the Prevention of Small Subcortical Strokes (SPS3) trials.25-27 Therefore, experts recommend aiming for a blood pressure target of less than 130/80 mmHg for individuals with DKD, provided it is well tolerated. However, a lower target may be considered for patients with significant albuminuria. Encouraging patients to monitor their blood pressure at home using a structured home blood pressure diary can enhance management.
All medications within the RAS inhibitor class exhibit a renoprotective effect, which goes beyond blood pressure reduction. This effect was first observed in trials of captopril in people with type 1 diabetes and later seen with losartan and irbesartan in patients with type 2 diabetes in the Irbesartan Diabetic Nephropathy Trial (IDNT) and Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial, respectively.28,29 These trials demonstrated a 16 to 20% relative risk reduction in developing ESKD, a result not observed with other antihypertensive classes used in those trials.
The recommended second-line agent for blood pressure control in patients with DKD is amlodipine, based on the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH). This showed CV benefits in patients prescribed an ACE inhibitor and amlodipine compared with an ACE inhibitor and hydrochlorothiazide combination.30
Reduce albuminuria and slow the decline in eGFR
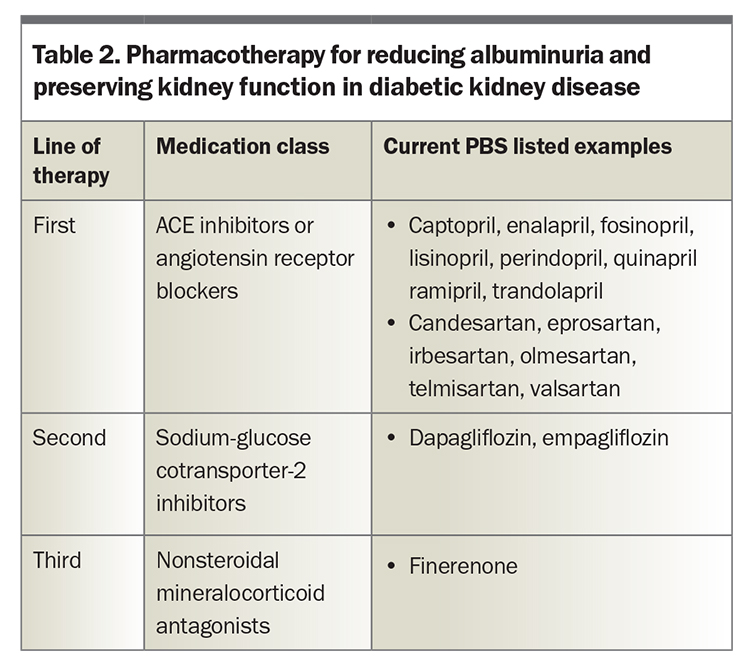
Although healthy individuals generally experience a gradual decline in GFR of between 0.2 and 1.0 mL/min/1.73m² per year, patients with DKD typically show a faster progression to ESKD that is proportional to the severity of albuminuria.31 Drugs that lower intraglomerular pressure contribute to a reduction in albuminuria and slow the decline in eGFR, which forms the basis for their renoprotective properties (Table 2).
RAS inhibitors and SGLT-2 inhibitors
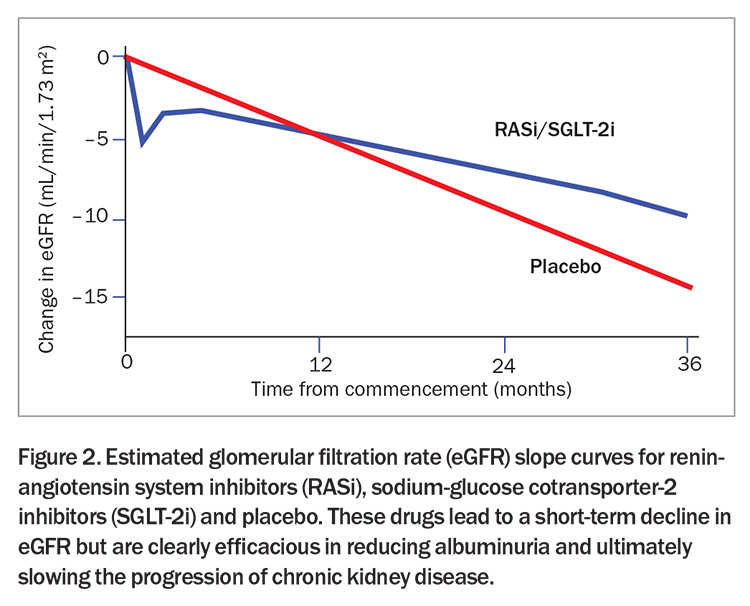
RAS inhibitors (ACE inhibitors and ARBs) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors work to counter the hyperfiltration that occurs in DKD. RAS inhibitors prevent efferent arteriolar vasoconstriction and SGLT-2 inhibitors increase afferent arteriolar vasoconstriction, collectively reducing intraglomerular pressure and hyperfiltration. An expected outcome of reducing glomerular pressure is an initial decrease in GFR followed by stabilisation (Figure 2). Importantly, this decline in GFR should not be misconstrued as acute kidney injury. Studies have shown that SGLT-2 inhibitors instead reduce the incidence of acute kidney injury and, therefore, the temporary drop in eGFR upon starting SGLT-2 inhibitors should not prompt discontinuation of either SGLT-2 inhibitors or RAS inhibitors.32
RAS inhibitors should be used as a first-line antihypertensive or if the patient has any degree of albuminuria (regardless of blood pressure). For individuals on alternative antihypertensives experiencing albuminuria or declining GFR, experts recommend switching to an ACE inhibitor or ARB and increasing to the maximally tolerated dose. The first captopril trial in patients with diabetic nephropathy showed a 50% reduction in the combined endpoint of death or ESKD.3 The goal is to achieve the maximum prescribed dose of RAS inhibitors, if tolerated. The Renoprotection of Optimal Antiproteinuric Doses (ROAD) study emphasised the importance of titrating to the maximally tolerated dose, showing a significantly lower incidence of the primary composite outcome (doubling of serum creatinine level, ESKD or death) in the group that underwent maximal titration.33
SGLT-2 inhibitors represent the biggest advancement in managing diabetic nephropathy since ACE inhibitors. The pleiotropic effects of SGLT-2 inhibitors are most evident in patients with CV disease. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG) trial showed a 38% relative risk reduction in death from CV causes and a 32% reduction in overall mortality with the use of empagliflozin in patients with type 2 diabetes.14,34 The Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial demonstrated a lower rate of CV death or hospitalisation due to heart failure.16 This CV benefit is likely a class effect, extending across heart failure exacerbations, hospitalisations and death.
Dapagliflozin and empagliflozin are PBS listed for adjunct therapy in patients diagnosed with heart failure (New York Heart Association class II, III or IV) with reduced ejection fraction (left ventricular ejection fraction ≤40%), with or without comorbid diabetes. Empagliflozin is also PBS listed for adjunct therapy in patients with heart failure with preserved ejection fraction. In patients diagnosed with renal impairment (with or without diabetes), dapagliflozin was shown to reduce the decline in eGFR, ESKD and death from renal or CV causes by 39%.15 Dapagliflozin is also PBS listed for CKD (as defined by a urine albumin to creatinine ratio ≥22.6 mg/mmol or eGFR 25 to 75 mL/min/1.73 m2), provided it is prescribed in combination with an ACE inhibitor or ARB. Full details are available on the PBS website. SGLT-2 inhibitors should be considered for all patients with DKD, regardless of glycaemic control, for CV and kidney risk reduction. Notably, SGLT-2 inhibitors are also efficacious in enhancing diuresis and for patients already taking a diuretic, the recommended dose of the diuretic should be reduced by 50%. Alternatively, the SGLT-2 inhibitor can be used at a standard dose as an additional therapy to support diuresis.
Dapagliflozin can be initiated in patients with an eGFR of 25 mL/min/1.73m2 or greater. Empagliflozin can be initiated in patients with type 2 diabetes and an eGFR of 30 mL/min/1.73 m2 or greater and in patients with heart failure (with or without diabetes) and an eGFR of 20 mL/min/1.73m2 or greater. Despite the impairments to glycaemic control as eGFR declines, the renoprotective properties of SGLT-2 inhibitors are maintained at low eGFRs. Once initiated, either SGLT-2 inhibitor should be continued until the patient commences dialysis or undergoes kidney transplantation. Although it is common practice to check creatinine levels four to six weeks following commencement of these medications, many clinicians skip this monitoring due to the expected acute decrease in eGFR (which stabilises within three months). In cases where eGFR decreases, it is recommended to repeat the assessment two to four weeks later. If eGFR decreases further, the patient should be referred to a nephrologist for further evaluation. If the decrease in renal function persists but remains stable, all medications should be continued and renal function should be rechecked in three to six months.
Mineralocorticoid antagonists
In patients with DKD and progressive albuminuria despite maximal therapy, steroidal mineralocorticoid antagonists (e.g. spironolactone) have been historically used. However, their use is often hindered by unwanted antiandrogen and oestrogen side effects as well as hyperkalaemia (especially as DKD progresses).
These unwanted side effects have led to the development of nonsteroidal mineralocorticoid antagonists such as finerenone. Finerenone is PBS listed for patients with type 2 diabetes and CKD with an eGFR of 25 mL/min/1.73 m2 or greater and a urine albumin to creatinine ratio of 22.6 mg/mmol or greater prior to initiating treatment with this drug. Patients must also be taking an ACE inhibitor or ARB and an SGLT-2 inhibitor, unless medically contraindicated. Finerenone can be continued until the patient commences dialysis or undergoes kidney transplantation. Full details are available on the PBS website.
Finerenone confers benefits beyond blood pressure control and demonstrates significantly lower rates of hyperkalaemia compared with steroidal mineralocorticoid antagonists. The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trials showed a 23% reduction in a composite kidney outcome (kidney failure, kidney death or doubling of creatinine level). Finerenone is a third-line option for managing patients with persistent albuminuria despite ACE inhibitor or ARB and SGLT-2 inhibitor treatment in patients with DKD.35-37 For patients who experience nonemergent hyperkalaemia in the setting of stage 3 and 4 CKD, the potassium binder patiromer works to lower serum potassium level and has recently been PBS listed. The patient must also be taking a RAS inhibitor and their condition must be inadequately controlled by a low potassium diet.
Reduce cardiovascular risk
Lipid management
DKD is associated with a twofold increased risk of developing CV disease, and sudden cardiac death is the leading cause of mortality in patients with ESKD.38 Although a comprehensive review of lipid management exceeds the scope of this article, it is important to recognise that patients who develop DKD experience the highest mortality risk from CV complications. The SHARP trial showed a 17% reduction in major atherosclerotic events for patients prescribed simvastatin 20 mg plus ezetimibe 10 mg daily. Notably, almost two-thirds of study participants had an eGFR of less than 30 mL/min/1.73 m2; however, this benefit has not been observed in patients undergoing dialysis (the reasons for which are complex).39
Experts recommend the use of statins in all patients, irrespective of their non-HDL or LDL cholesterol levels. The ideal targets are an LDL cholesterol level below 1.8 mmol/L or non-HDL cholesterol level below 2.5 mmol/L. In cases where these targets are not achieved with statin monotherapy, specialists suggest additional therapy with ezetimibe followed by a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) if necessary. However, caution is advised in using high-dose statin therapy in patients with stage 5 CKD because of the associated risk of rhabdomyolysis.
Glycaemic control for patients with high cardiovascular risk
The two diabetes drug classes that have demonstrated substantial reduction in CV events are SGLT-2 inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists. Therefore, the third-line therapy of most specialists for the management of DKD is a GLP-1 receptor agonist. Multiple trials not only show significant CV benefits but also improvement in urine albumin to creatinine ratios.40 Although SGLT-2 inhibitors have become the mainstay treatment for DKD, GLP-1 receptor agonists have a distinct role, particularly in patients with obesity.
The PBS requires that patients be on insulin or both metformin and a sulfonylurea with an HbA1c greater than 7.0 mmol/mol prior to initiating a GLP-1 receptor agonist. Due to global shortages, the Pharmaceutical Benefits Advisory Committee recommended the restriction of GLP-1 receptor agonists to patients who are contraindicated, intolerant or inadequately responsive to SGLT-2 inhibitors.41
Future of DKD therapy
Over the past decade, there have been remarkable advances in the treatment of diabetes and DKD, offering great promise for patients. The introduction of new therapies has considerably widened the range of available treatments, presenting an extensive arsenal for medical professionals. Ideally, the goal is now to ensure universal access to these groundbreaking therapies and allow all patients to reap the maximum benefits of these advances.
The future appears promising, with numerous agents targeting diverse pathways in diabetes and DKD. Expected developments include the wider availability of the combined GLP-1 receptor and glucose-dependent insulinotropic polypeptide agonist tirzepatide, which has shown impressive results for weight loss. Although secondary analyses demonstrate benefits to kidney function, these require confirmation in future studies that include primary kidney endpoints.42,43 Although still to be tested in DKD, this class of drug holds great potential.
Additionally, ongoing trials are exploring the combination of endothelin receptor antagonists with SGLT-2 inhibitors and therapies involving novel signalling pathways are in development. These agents show potential in targeting critical pathways specific to DKD, offering the possibility of significant advances in treatment options in the near future.
Conclusion
Early diagnosis and intervention significantly improve outcomes in patients with DKD. Management strategies should focus on early intervention, following the principles of modifying lifestyle factors, optimising glycaemic control, reducing CV risk factors and preventing any further deteriorations or complications of CKD. Novel agents will soon be accessible with the potential to reduce the substantial morbidity and mortality associated with DKD. ET
COMPETING INTERESTS: None.
References
1. Australia and New Zealand Dialysis and Transplant Registry. Incidence of kidney failure with replacement therapy. Adelaide: The Registry; 2022. Available online at: http://www.anzdata.org.au (accessed January 2024).
2. Australian Institute of Health and Welfare. Chronic kidney disease: Australian facts. Canberra: AIHW; 2023. Available online at: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/about (accessed January 2024).
3. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993; 329: 1456-1462.
4. Pollock CA, Bostrom TE, Dyne M, Györy AZ, Field MJ. Tubular sodium handling and tubuloglomerular feedback in compensatory renal hypertrophy. Pflügers Archiv 1992; 420, 159-166.
5. Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol 1991; 260, 946-952.
6. Mazzucco G, Bertani T, Fortunato M, et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis 2002; 39, 713-720.
7. Rossing P, Caramori M, Chan J, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022; 102, S1-S127.
8. Suarez MLG, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes 2013; 4: 245.
9. Chandy A, Pawar B, John M, Isaac R. Association between diabetic nephropathy and other diabetic microvascular and macrovascular complications. Saudi J Kidney Dis 2008; 19: 924-928.
10. Lean MEJ, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019; 7: 344-355.
11. Chaiton M, Diemert L, Cohen J, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open 2016; 6: e011045.
12. Mix T-C, Brenner R, Cooper M, et al. Rationale—Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. Am Heart J 2005; 149: 408-413.
13. Perkovic V, Jardine M, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 280: 2295-2306.
14. Zinman B, Wanner C, Lachin J, et al; EMPA-REG Outcome Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 2117-2128.
15. Heerspink H, Stefánsson B, Correa-Rotter R, et al; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
16. Wiviott SD, Raz I, Bonaca M, et al; DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347-357.
17. EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2022; 388: 117-127.
18. Mann J, Ørsted D, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839-848.
19. Rossing P, Baeres F, Bakris G, et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant 2023; 38: 2041-2051.
20. Shaman A, Bain S, Bakris G, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 2022; 145: 575-585.
21. Marso S, Daniels G, Brown-Frandsen, et al; LEADER Trial Investigators. ‘Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
22. Rosenstock J, Kahn S, Johansen O, et al; CAROLINA Investigators. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA 2019; 322: 1155-1166.
23. Rosenstock J, Perkovic V, Johansen O, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019; 321: 69-79.
24. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 1999; 48: 643.
25. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1575-1585.
26. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103-2116.
27. Benavente OR, Coffey CS, Conwit R, et al; SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382: 507-515.
28. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861-869.
29. Lewis EJ, Hunsicker LG, Clarke WE, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851-860.
30. Jamerson K, Weber M, Bakris G, et al; ACCOMPLISH trial investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359: 2417-2428.
31. Noronha IL, Santa-Catharina GP, Andrade L, et al. Glomerular filtration in the aging population. Front Med (Lausanne) 2022; 9: 769329.
32. Heerspink HJL, Cherney DZI. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol 2021; 16: 1278.
33. Hou FF, Xie D, Zhang X, et al. Renoprotection of optimal antiproteinuric doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 2007; 18: 1889-1898.
34. Baigent C, Emberson J, Haynes R, et al; Nuffield Department of Population Health Renal Studies Group. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022; 400: 1788-1801.
35. Agarwal R, Filippatos G, Pitt B, et al; FIDELIO-DKD and FIGARO-DKD Investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43: 474-484.
36. Bakris GL, Agarwal R, Anker S, et al; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
37. B. Pitt B, Filippatos G, Agarwal R, et al; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252-2263.
38. Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis 2014; 21: 273-280.
39. Khan SU, Khan MU, Virani SS, et al. Efficacy and safety for the achievement of guideline-recommended lower low-density lipoprotein cholesterol levels: a systematic review and meta-analysis. Eur J Prev Cardiol 2022; 28: 2001-2009.
40. Yu JH, Park SY, Kim NH, Seo JA. GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions. Kidney Res Clin Pract 2022; 41: 136.
41. Pharmaceutical Benefits Scheme. Recommendations made by the PBAC – July 2023. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/recommendations-made-by-the-pbac-july-2023 (accessed January 2024).
42. Jastreboff A, Aronne L, Ahmad N, et al; SURMOUNT-1 Investigators. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205-216.
43. Frías J, Davies M, Rosenstock J, et al; SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.