Cardiovascular risk in type 2 diabetes. An evidence-based approach to management

Type 2 diabetes is associated with increased cardiovascular risk, with traditional management focusing on lifestyle modifications and management of lipid levels and blood pressure. The management of cardiovascular risk in type 2 diabetes has evolved rapidly in recent years, with strong clinical evidence supporting lower lipid targets and the use of newer antihyperglycaemic agents, which have shown cardiovascular benefits independent of their glycaemic effects.
- Cardiovascular disease is a major health issue for people living with type 2 diabetes.
- Early and aggressive guideline-based management of modifiable cardiovascular risk factors improves health outcomes.
- Stratification of cardiovascular risk involves the use of CVD risk calculators, thorough clinical assessment and, in selected patients, a coronary artery calcium score.
- GPs have an essential role in guiding and educating patients on the importance of cardiovascular risk management.
- As part of routine diabetes management, GPs and all involved in the care of people with diabetes, should regularly reassess cardiovascular risk factors and intensify treatment as required.
- Sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists improve cardiovascular outcomes and should be considered for all people with type 2 diabetes, particularly those at high risk.
Type 2 diabetes is a complex, chronic health condition affecting over 500 million people globally and 1.3 million people in Australia.1,2 Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in people with diabetes.3 Atherosclerotic cardiovascular disease (ASCVD) includes coronary heart disease (CHD), cerebrovascular disease and peripheral arterial disease. Type 2 diabetes is frequently associated with multiple comorbidities that increase ASCVD risk, including hypertension, dyslipidaemia, smoking and obesity. Optimal cardiovascular management involves an individualised, multifactorial approach that appropriately targets modifiable ASCVD risk factors. This article discusses the current recommendations for ASCVD risk management in type 2 diabetes, highlighting recent changes.
Cardiovascular risk assessment
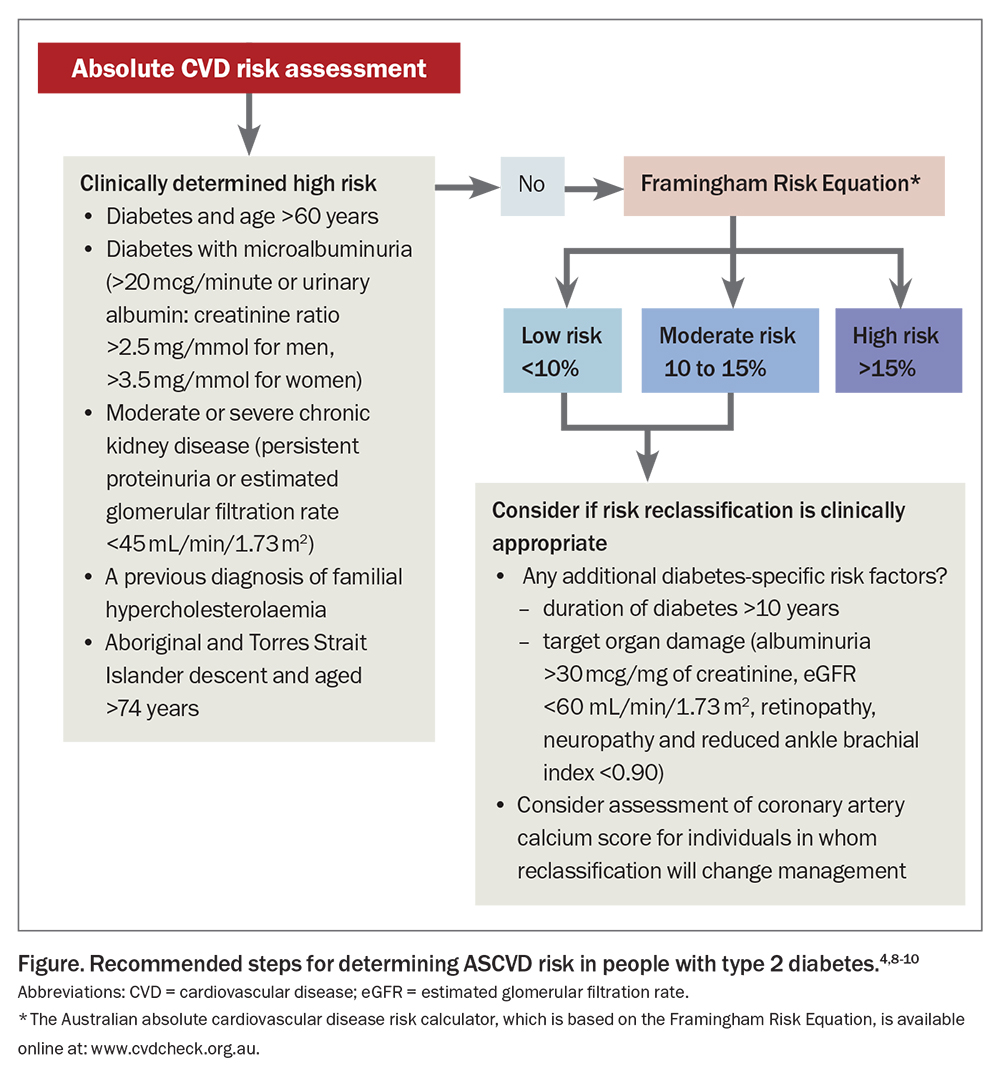
Calculating a patient’s CVD risk helps determine the need for and intensity of therapy. Risk can be calculated using the Australian absolute cardiovascular disease risk calculator, which is based on the Framingham Risk Equation and estimates the risk of a patient experiencing a CVD event within the next five years (https://www.cvdcheck.org.au). People older than 65 years of age with type 2 diabetes are considered to be at high CVD risk according to the current Australian Guidelines from the National Vascular Disease Prevention Alliance (NVDPA).4 It is therefore not necessary to calculate risk in these patients. Chronic kidney disease is also recognised as an independent risk factor for CVD. Diabetic nephropathy affects about 20 to 40% of people with diabetes.5 Those with diabetes and microalbuminuria (urinary albumin excretion >20 mcg/minute or urinary albumin to creatinine ratio >2.5 mg/mmol for men and >3.5 mg/mmol for women) are also clinically classified as having a high risk for CVD.4 Importantly, CVD risk in socioeconomically disadvantaged and ethnic groups, including the Aboriginal and Torres Strait Islander population, is underestimated. Any calculated estimate of risk should be considered the minimum in these groups. The Figure outlines the method for risk calculation, including clinical determinants of risk.4
In people with diabetes, this simplistic approach to risk assessment inadequately estimates risk, as the influence of disease duration and presence of diabetic complications are not considered. It can also fail to identify those with silent ischaemia.6,7 Several international guidelines now consider additional diabetes-specific risk factors when determining CVD risk.8,9 The National Heart Foundation of Australia recommends using the coronary artery calcium score to further delineate the CVD risk for moderate- and selected low-risk patients in whom reclassification would change management.10 An Agatston score (density of calcification in a given coronary artery) over 99 Agatston units indicates high absolute risk with the need for preventative treatment. The score is validated in people aged between 40 and 70 years.
Cardiovascular risk management
An individualised, patient-centred approach with shared decision-making is recommended for ASCVD risk management. GPs should discuss with patients their diagnosis, associated health risks and management goals. This will assist patients to take ownership of their long-term diabetes management and is likely to result in improved patient outcomes.
Lifestyle modifications
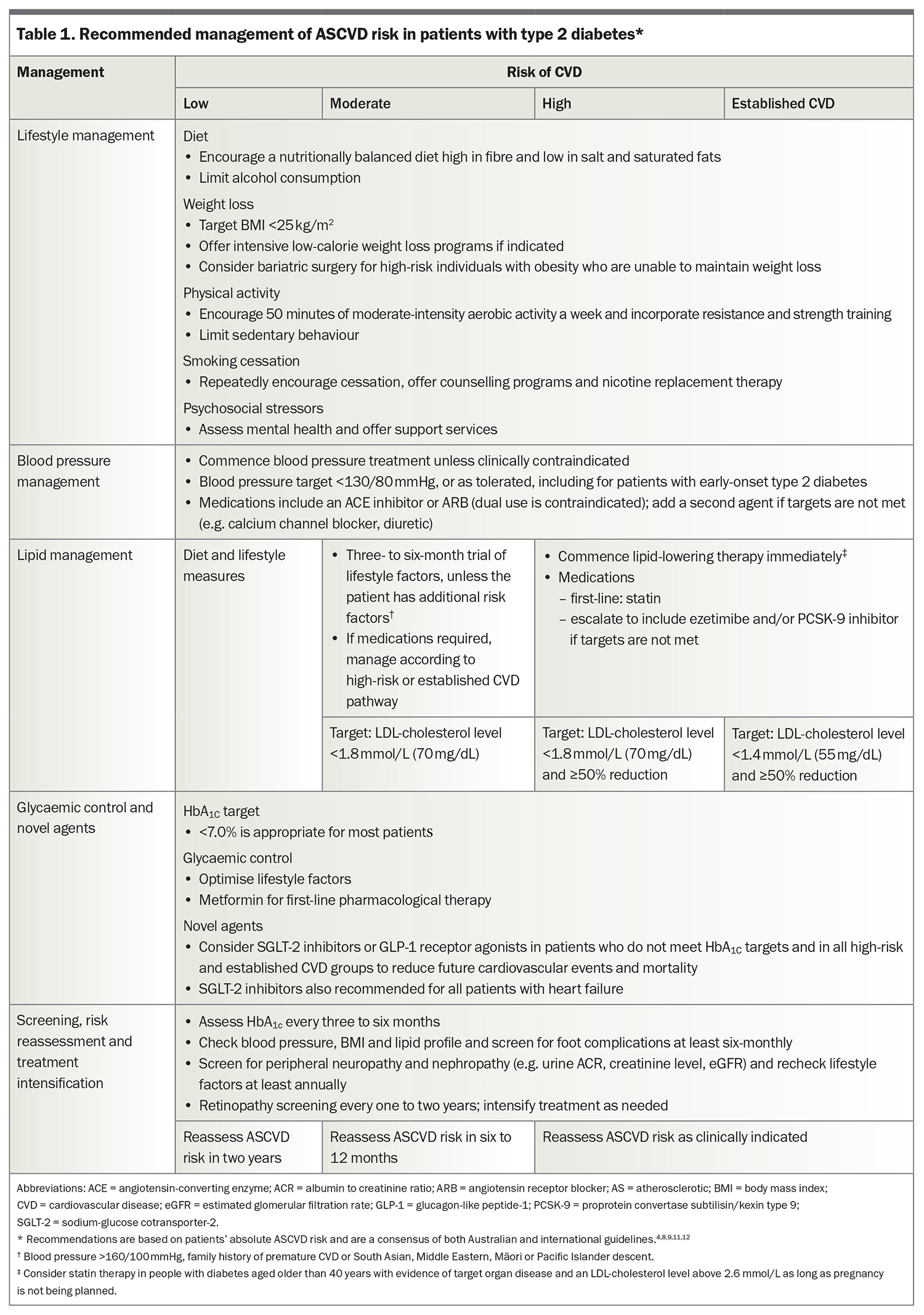
Lifestyle optimisation is appropriate for all people with diabetes to improve ASCVD risk factors. A brief summary follows, with detailed advice available in The Royal Australian College of General Practitioners’ Management of type 2 diabetes: A handbook for general practice and Table 1.4,8,9,11,12
Diet and exercise
A nutritionally balanced, low-calorie diet with increased physical activity is recommended for weight loss. The Look AHEAD (Action for Health and Diabetes) trial found that patients who followed an intensive diet and exercise intervention program improved the likelihood of achieving their glycaemic, blood pressure and lipid targets, although there was no impact on overall survival or cardiovascular mortality.13 Involving a dietician and exercise physiologist should always be considered. Weight loss medications and metabolic or bariatric surgery can also be considered.11
Smoking and vaping
Smoking contributes to the progression of atherosclerosis and CVD. Smoking cessation should be strongly and repeatedly encouraged. A combination of medications and multi-session behavioural interventions, such as Quitline, gives patients the best chance of successfully quitting.14,15 Vaping should also be discouraged because of the potential health risks and increased likelihood of smoking.11,16
Blood pressure management
Treatment of hypertension reduces cardiovascular events and all-cause mortality in people with diabetes.17 Patients should have their blood pressure monitored at each review. A blood pressure target below 130/80 mmHg is recommended for all patients with diabetes clinically safe, regardless of their risk category.4,8,9 An angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) is recommended as first-line antihypertensive therapy, given their renal-protective effects.8,18,19 If monotherapy does not sufficiently reduce blood pressure, a second agent from a different pharmacological class should be added, such as a calcium channel blocker, low-dose thiazide or thiazide-like diuretic.10 ACE inhibitors and ARBs should not be coprescribed.20 Patients should also be monitored for adverse side effects including hypotension, syncope, acute kidney injury and electrolyte disturbances.
Lipid management
LDL cholesterol
Accumulation of LDL and other cholesterol-rich lipoproteins in arterial walls promotes plaque formation and atherosclerosis. Strong clinical evidence supports the efficacy of statins in people with diabetes for both primary and secondary prevention.21-23 A meta-analysis of 170,000 patients (with and without diabetes) involved in 26 randomised clinical trials of statins showed that every 1 mmol/L reduction in LDL cholesterol translated to a 10% reduction in all-cause mortality.24 With more effective and clinically proven therapies becoming available, recommended LDL targets have continued to fall over time. From a safety perspective, there is no evidence that a low LDL-cholesterol level is harmful.25
Statins are first-line pharmacological therapy.8 Treatment should aim to reduce LDL-cholesterol levels by at least 50% from baseline, with a target below 1.8 mmol/L for those at high risk of CVD and below 1.4 mmol/L for patients with known CVD. If these targets are not able to be achieved on statin alone, or statin use is contraindicated, ezetimibe or a proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitor should be added.8 PCSK-9 therapies are now more firmly indicated in people with diabetes not achieving appropriate LDL-cholesterol levels and represent an important improvement in lipid management.26 A new antisense PCSK-9 molecule may soon be available and could represent a major advance in the management of hyperlipidaemia.27
The recent CLEAR Outcomes trial also showed promising results for bempedoic acid, an adenosine triphosphate citrate lyase inhibitor. Treatment in statin-intolerant patients resulted in reduced LDL-cholesterol levels and a lower risk of major adverse cardiovascular events.28
For young adults (aged <40 years) with type 2 diabetes, absolute CVD risk calculators may not accurately capture the patient’s true risk. Early and aggressive treatment of cardiovascular risk factors is recommended to reduce the lifetime risk of coronary artery disease in these patients. The European Society of Cardiology Guidelines recommend considering commencing statins in this group if there is evidence of target organ damage, such as microalbuminuria, retinopathy, neuropathy or an LDL-cholesterol level above 2.6 mmol/L.8 Australian Guidelines recommend initiating lipid-lowering medication for people aged 18 to 30 years with type 2 diabetes and LDL-cholesterol levels above 3.4 mmol/L, with a target below 2.6 mmol/L.29
Familial hypercholesterolaemia should be considered in patients with a total cholesterol level over 7.5 mmol/L. If suspected, genetic analysis can be undertaken allowing for family tracing and the early identification of this disorder in asymptomatic relatives.
Patients who develop diabetes after the age of 70 years will have less lifetime benefit from therapy and this may not be indicated. The need for therapy in this group should be determined on an individual basis and, if started, lower doses are indicated and monitoring for renal impairment and drug interactions is important.
Triglycerides
Pharmacotherapy is recommended for patients with type 2 diabetes at high risk of CVD and with fasting triglyceride levels above 2.3 mmol/L, despite lifestyle optimisation. If the LDL-cholesterol level is also elevated, statins are the first-line choice. Fibrates and omega-3 fatty acids, at a recommended dose of 4 g per day, are second-line therapies that can be used in conjunction with statins. However, these agents are the preferred therapies either alone or in combination to treat severe hypertriglyceridaemia. In this situation, the aim of therapy is to lower the risk of pancreatitis, not CVD. The use of fibrates has also been shown to reduce the rate of progression of retinal disease in people with diabetes.30,31
Glycaemic control
Optimal glycaemic control in type 2 diabetes is associated with a reduced risk of microvascular complications (retinopathy, nephropathy and neuropathy). A glycated haemoglobin target below 7% is recommended for most adults.32 Lifestyle or dietary changes alone are often insufficient. Metformin remains the first-line treatment for patients with newly diagnosed type 2 diabetes and is generally well tolerated, with a low risk of hypoglycaemia.11 It has been shown to reduce the rate of CVD, although only in a small number (n = 342) of overweight patients (BMI >31 kg/m2) after many years (10 years) of therapy.33 However, several larger clinical trials failed to show a cardiovascular benefit from tight glycaemic control and in some studies, intensive glycaemic control was associated with increased hypoglycaemic events and mortality.34
Second-line antihyperglycaemic agents
If glycaemic targets have not been met on metformin, additional therapies should be added. In Australia, this includes sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl-peptidase-4 (DPP-4) inhibitors, sulfonylureas (SUs), thiazolidinediones (TZDs) and insulin. DPP-4 inhibitors, SUs, TZDs and insulin have not been shown to reduce the rate of CVD events despite improving glycaemic control, with aggressive therapy with these agents increasing cardiovascular morbidity and mortality.35,36
A meta-analysis of over 400,000 patients with type 2 diabetes showed that SGLT-2 inhibitors and GLP-1 receptor agonists had cardiovascular benefits, including reduced all-cause mortality, cardiovascular mortality, nonfatal myocardial infarction and kidney failure.37 Given the high burden of CVD in patients with type 2 diabetes, several guidelines now recommend including at least one of these agents in the management of type 2 diabetes in patients at high risk of CVD or with established ASCVD, regardless of their glycated haemoglobin level.8,9,11,12
SGLT-2 inhibitors
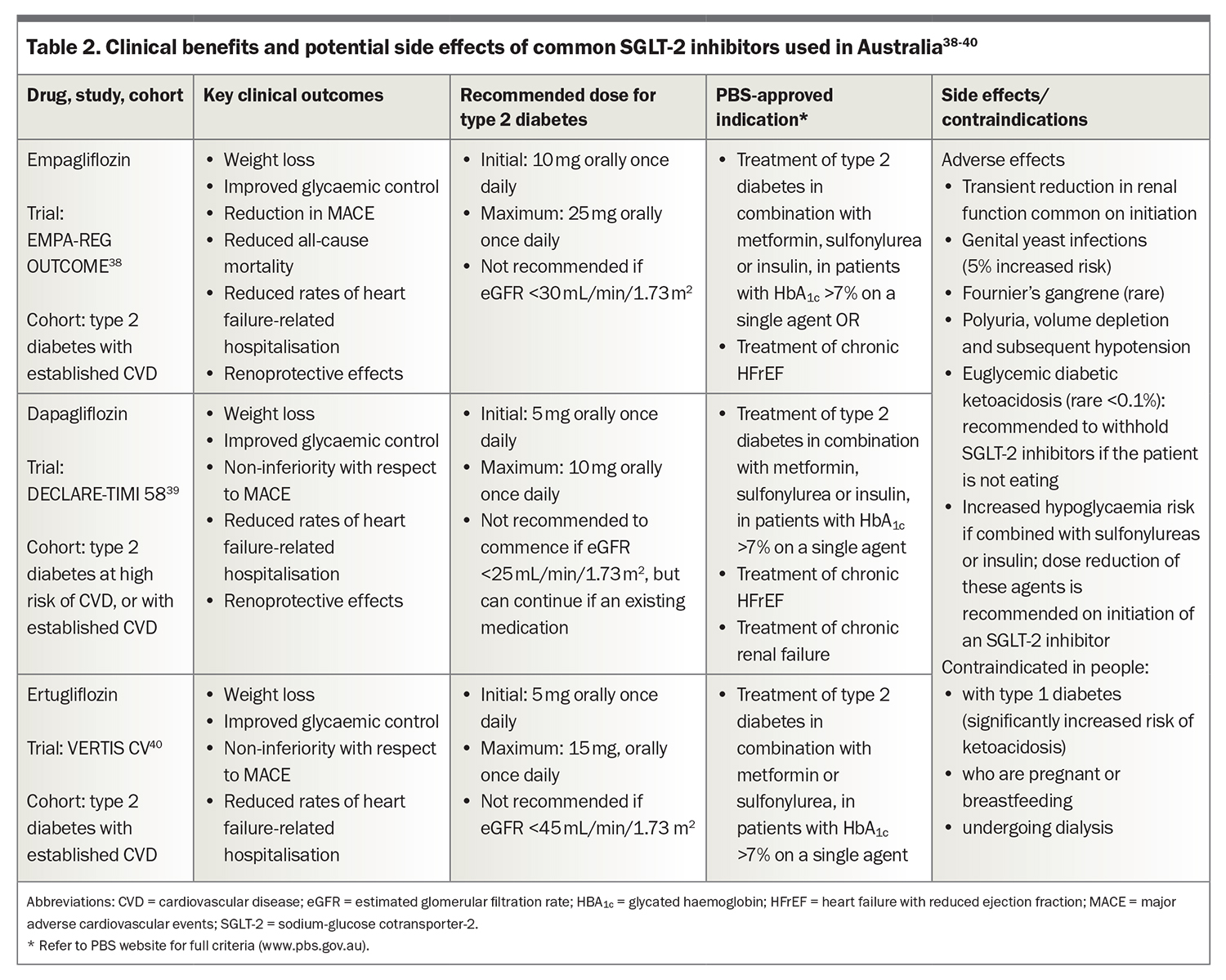
SGLT-2 inhibitors include the oral agents empagliflozin, dapagliflozin and ertugliflozin that act by reducing renal tubular glucose reabsorption and increasing glycosuria. The EMPA-REG (Empagliflozin, Cardiovascular Outcome and Mortality in Type 2 Diabetes) study was the first trial to show a reduced rate of cardiovascular mortality and reduction in hospitalisation for heart failure.38 Subsequent studies with other SGLT-2 inhibitors have confirmed some or all of these benefits in various populations, including patients without diabetes.36 Importantly, all these therapies slow the rate of deterioration of renal function in patients with type 2 diabetes when compared with placebo.
Potential side effects of SGLT-2 inhibitors are noted in Table 2.38-40 SGLT-2 inhibitors alone or in combination with metformin do not cause hypoglycaemia, but will potentiate this risk when added to insulin or sulfonylureas. The dose of those therapies may need to be reduced by 20 to 50%.
GLP-1 receptor agonists
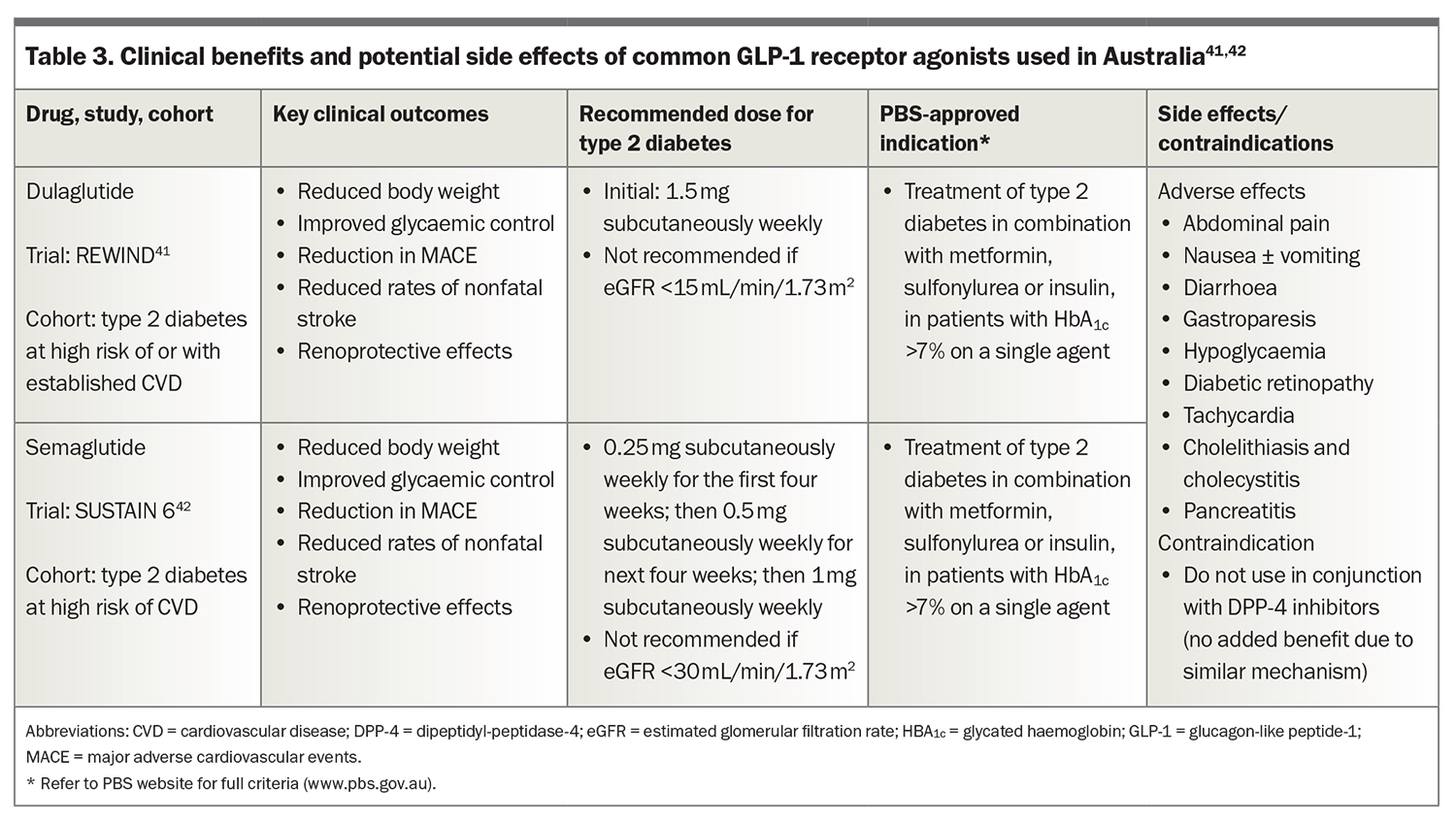
The GLP-1 receptor agonists liraglutide, semaglutide and dulaglutide are available in Australia. The latter two are PBS-listed and most commonly used and their potential side effects are summarised in Table 3.41,42 They are administered by weekly subcutaneous injection. GLP-1 receptor agonists mimic endogenous GLP-1 to enhance insulin secretion and inhibit glucagon secretion from pancreatic islet cells. GLP-1 receptor agonists have a central effect on the hypothalamus to suppress appetite. This may be their most important therapeutic action. GLP-1 receptor agonists also delay gastric emptying, further contributing to early satiety. They have a significant effect on glycaemic control and can result in meaningful weight loss.
Importantly, they are also associated with cardiovascular benefits. The first study to demonstrate a CVD benefit was the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial.43 Lower rates of cardiovascular and all-cause mortality, with nonsignificant reductions in nonfatal myocardial infarction, nonfatal stroke and heart failure-related hospitalisation were observed. Subsequently, trials with semaglutide and dulaglutide confirmed significantly reduced rates of cardiovascular events in people with diabetes with and without established CVD.41,42
Several newer therapies targeting the GLP-1 receptor are in development and potentially may have greater therapeutic benefit. Tirzepatide is one of these newer molecules and has recently been approved by the TGA. It targets both the GLP-1 and glucose-dependent insulinotropic polypeptide receptors. A phase three trial comparing tirzepatide with semaglutide (1 mg) showed tirzepatide to be superior in both glycaemic control and average weight loss. A 15 mg dose of tirzepatide resulted in average weight loss of 11.2 kg after 40 weeks, compared with a 5.7 kg loss with semaglutide.44 A CVD outcome trial is underway at several centres in Australia. The study is ongoing and results are not available at the time of writing this review.
Abdominal pain, nausea, vomiting and diarrhoea are known side effects of GLP-1 receptor agonists. Starting patients at an initial low dose, with slow up-titration, aids tolerance. DPP-4 inhibitors and GLP-1 receptor agonists both function to enhance GLP-1 activity and, therefore, should not be given in combination.
The important unanswered clinical question is whether the cardiovascular benefits of a long-acting GLP-1 receptor agonist used in combination with an SGLT-2 inhibitor will provide additive CVD benefit. This is unlikely to be answered in an appropriately conducted randomised prospective clinical trial. However, the mechanism of action of the two drug classes is different, suggesting that the benefit is likely to be additive. An additive effective on glycaemic control has been confirmed, and their combined use for glycaemic control is justified in appropriate clinical situations.45
Conclusion
Aggressive management of CVD risk is essential to prevent future cardiovascular events in people with type 2 diabetes. A multifactorial approach, with early treatment of CVD risk factors, achieves the best outcomes. Antihyperglycaemic therapies that reduce CVD risk, such as SGLT-2 inhibitors and GLP-1 receptor agonists, possibly in combination, are now part of established treatment. ET
COMPETING INTERESTS: Dr Wilkinson: None. Associate Professor d’Emden has served on national advisory boards for Eli Lilly, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Pfizer and Novartis; and is Principle Investigator on many of the major CVD outcome studies in diabetes, including EMPA-REG, LEADER, SURPASS-2 and SURPASS-CVD.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022; 183:109119.
2. Australian Bureau of Statistics (ABS). National Health Survey: 2020-2021. In: Statistics ABo, eds. ABS; Canberra, 2020. Available online at: https://www.abs.gov.au/methodologies/national-health-survey-first-results-methodology/2020-21 (accessed April 2023).
3. Rawshani A, Rawshani A, Franzen S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017; 376: 1407-1418.
4. National Vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk. Melbourne: National Stroke Foundation; 2012. Available online at: https://informme.org.au/media/cuzjrcgz/absolutecvd_gl_webready.pdf (accessed April 2023).
5. Mok Y, Ballew SH, Matsushita K. Chronic kidney disease measures for cardiovascular risk prediction. Atherosclerosis. 2021; 335: 110-118.
6. Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care 2004; 27: 1954-1961.
7. Scognamiglio R, Negut C, Ramondo A, Tiengo A, Avogaro A. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol 2006; 47: 65-71.
8. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227-3237.
9. Arnett DK, Khera A, Blumenthal RS. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Part 1, Lifestyle and Behavioral Factors. JAMA Cardiol 2019; 4: 1043-1044.
10. Jennings GL, Audehm R, Bishop W, et al. National Heart Foundation of Australia: position statement on coronary artery calcium scoring for the primary prevention of cardiovascular disease in Australia. Med J Aust 2021; 214: 434-439.
11. The Royal Australian College of General Practitioners. Management of type 2 diabetes: a handbook or general practice. RACGP and and Diabetes Australia; Melbourne; 2020.
12. ElSayed NA, Aleppo G, Aroda VR, et al. Erratum. 10. Cardiovascular disease and risk management: Standards of Care in Diabetes-2023. Diabetes Care 2023; 46(Suppl.1): S158-S190. Diabetes Care. 2023; 46(4): 898.
13. Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369: 145-154.
14. Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev 2015; (10): CD009670.
15. Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017; 3(3): CD001292.
16. Echeagaray O, Savko C, Gallo A, Sussman M. Cardiovascular consequences of vaping. Curr Opin Cardiol 2022; 37: 227-235.
17. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016; 352: i717.
18. Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Renoprotective effects of renin-angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta-analysis. Diabetologia 2012; 55: 566-578.
19. Wang K, Hu J, Luo T, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res 2018; 43: 768-779.
20. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372: 547-553.
21. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364: 685-696.
22. Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl 2004; 5: 81-87.
23. Violi F, Micheletta F, Iuliano L, Study MBHP. MRC/BHF Heart Protection Study. Lancet 2002; 360: 1782-1783; author reply 3-4.
24. Cholesterol Treatment Trialists Collabration; Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670-1681.
25. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267-1278.
26. Simons LA. An updated review of lipid-modifying therapy. Med J Aust 2019; 211: 87-92.
27. Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020; 382: 1507-1519.
28. Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant iatients. N Engl J Med 2023; 388: 1353-1364.
29. Wong J, Ross GP, Zoungas S, et al. Management of type 2 diabetes in young adults aged 18-30 years: ADS/ADEA/APEG consensus statement. Med J Aust 2022; 216: 422-429.
30. ACCORD Study Group; ACCORD Eye Study Group; Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010; 363: 233-244.
31. Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007; 370: 1687-1697.
32. Cheung NW, Conn JJ, d’Emden MC, et al. Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Med J Aust 2009; 191: 339-344.
33. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854-865.
34. Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: now that the dust is settling from large clinical trials. Ann N Y Acad Sci 2013; 1281: 36-50.
35. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419-430.
36. Mosenzon O, Leibowitz G, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care 2017; 40: 69-76.
37. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021; 372: m4573.
38. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117-2128.
39. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347-357.
40. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425-1435.
41. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394: 121-130.
42. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375: 1834-1844.
43. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
44. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
45. Li C, Luo J, Jiang M, Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol 2022; 13: 838277.