Concurrent management of type 2 diabetes and obesity

Type 2 diabetes is prevalent in Australia, with most diagnosed patients also being affected by comorbid obesity. Weight loss can mitigate otherwise substantial risk in people with obesity and diabetes. Strategies for the management of weight in patients with type 2 diabetes include lifestyle modifications, pharmacotherapy and surgery.
- The prevalence of both diabetes and obesity is rapidly increasing in Australia.
- Weight loss improves the core metabolic features common to both conditions and reduces morbidity and mortality.
- Lifestyle interventions and pharmacotherapy should be introduced early.
- Glucagon-like peptide-1 receptor agonists are among the most effective medications to concurrently manage diabetes and obesity.
- Surgical intervention is a treatment option for the minority of people with diabetes and obesity.
Diabetes occurs in 5% of people in Australia, with almost 1.3 million people in 2021 diagnosed with type 2 diabetes. Diabetes contributed to 11.2% of all deaths in 2021.1 Obesity is one of the leading risk factors for developing type 2 diabetes, and almost 80 to 90% of people with type 2 diabetes have overweight or obesity.2 Obesity has become an increasing problem worldwide, with about 67% of adults in Australia having a body mass index (BMI) in either the overweight (25 to 29.9 kg/m2) or obesity (≥30 kg/m2) class.3 As the prevalence of obesity increases, there is also a rise in associated obesity-related complications, leading to further morbidity and mortality. These obesity-related complications include hypertension, hyperglycaemia, cardiovascular (CV) disease, type 2 diabetes, dyslipidaemia, airway disease, obstructive sleep apnoea, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, gastro-oesophageal reflux disease, urinary stress incontinence and osteoarthritis.4-6
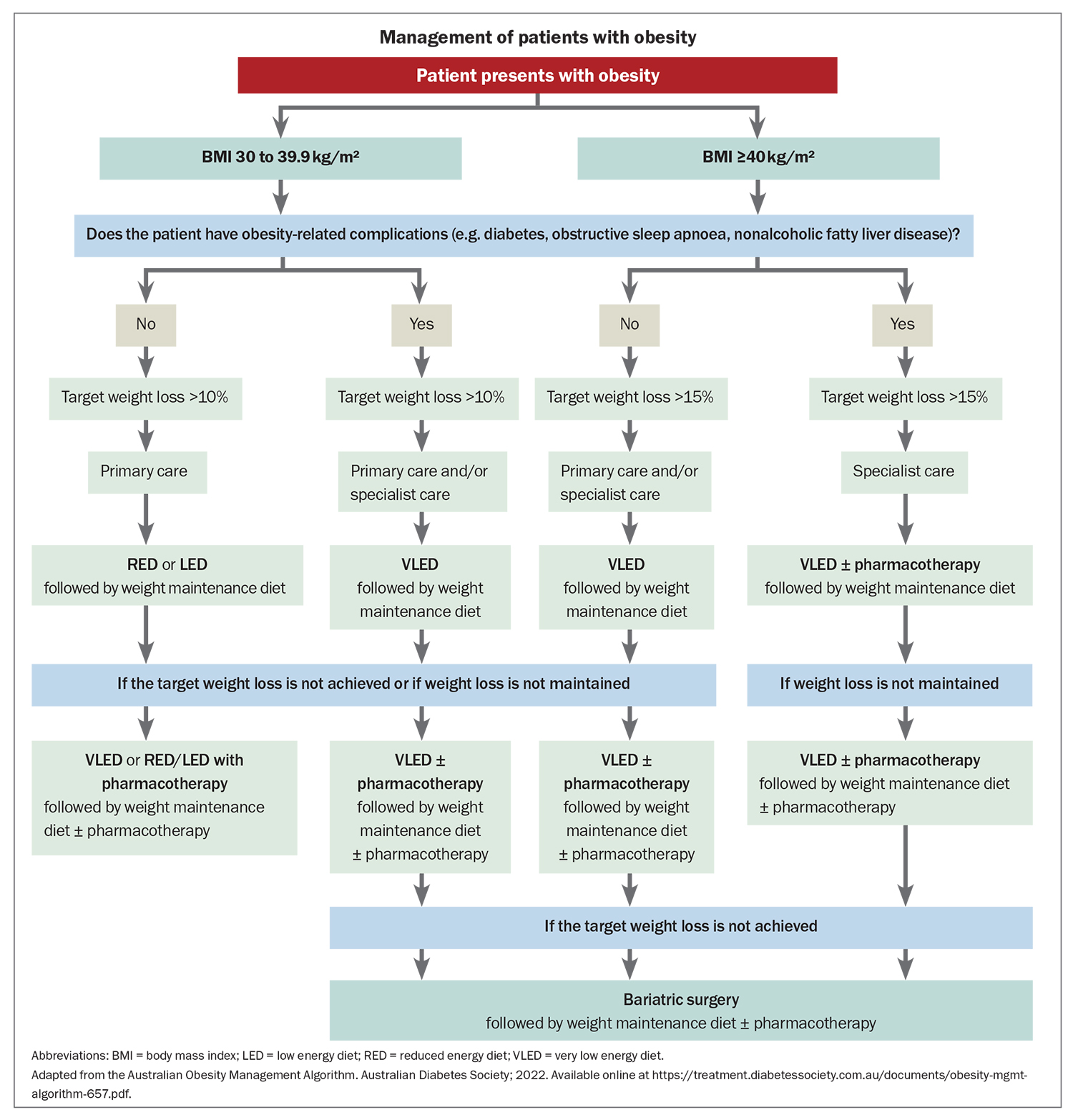
Compared with people who have a BMI within a healthy range, those with obesity have a significantly increased risk of obesity-related comorbidities; women have a 12.4 increased risk of developing type 2 diabetes, almost double that seen in men.7 In the context of obesity, women also have a higher risk of developing coronary artery disease, which is especially concerning given CV disease is the leading cause of death in both men and women.5 The rate of other complications is just as alarming, with more than one in 20 cancer cases directly related to patients being overweight or obese.6 Obesity is the second most common modifiable risk factor (after smoking) for developing cancer. Therefore, maintaining a healthy weight could be crucial for the primary prevention of a large disease burden in the future (Flowchart).
Benefits of weight loss
Weight loss can mitigate otherwise substantial risk in people with obesity and diabetes, with even modest reductions in weight (<5%) leading to improvements in hypertension and hyperglycaemia.8 A 5 to 10% weight loss is associated with a reduction of intrahepatic lipids in metabolic-associated fatty liver disease; improvement in forced expiratory volume at one second in those with asthma and airway disease; reduction in triglycerides, increase in high-density lipoprotein cholesterol, reduction in non-high-density lipoprotein cholesterol; improvements in ovulation and regularisation of menses, particularly in those women affected by polycystic ovarian syndrome; and prevention of type 2 diabetes.8,9 A 10% weight loss also results in a reduction in all-cause mortality.10 More progressive weight loss (>10%) significantly improves metrics associated with osteoarthritis (including pain, walking distance and quality of life scores), gastroesophageal reflux disease, obstructive sleep apnoea, nonalcoholic steatohepatitis and heart failure with preserved ejection fraction. Furthermore, achieving greater than 10% weight loss is associated with reductions in CV disease mortality and increased rates of type 2 diabetes remission, especially when the duration of the latter is short (Box).8,10-12
Therapeutic strategies for diabetes and obesity management
Lifestyle intervention
The Diabetes Remission Clinical Trial (DiRECT) demonstrated that lifestyle intervention implemented in general practice for people with type 2 diabetes can lead to meaningful weight loss and disease remission.8 After an initial three-month period of a reduced calorie diet (about 850 kcal/day) and a further six-week food introduction phase, participants were randomised to either standard care or a structured program providing individualised dietary advice and an exercise program. At one year, 46% of participants in the intervention arm were in remission (defined as a gylcated haemoglobin [HbA1c] <6.5 mmol/mol without medications) and 24% had achieved the target 15 kg weight loss or more. Although the number of participants who maintained sustained weight loss reduced over the next year, there was still evidence of a benefit, with 36% of those in the intervention arm remaining in type 2 diabetes remission. This pivotal trial provides evidence, that beyond a drop in body weight, lifestyle intervention can facilitate remission of type 2 diabetes.
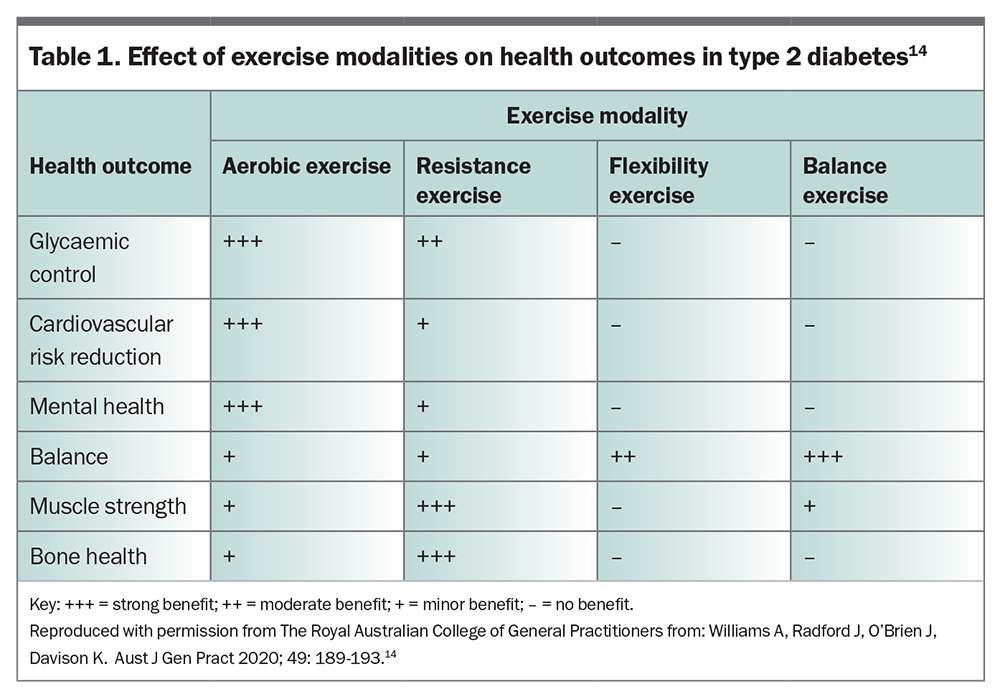
Physical activity should always be encouraged in conjunction with dietary interventional strategies, with key objectives tailored to individuals and available resources. Standard recommendations include 150 minutes of moderate-to-vigorous intensity exercise per week; however, exercise physiologists recommend avoiding a prescribed amount of physical activity or setting unacheivable goals and focusing instead on activities the patient enjoys, such as dancing, yoga or swimming. There is strong evidence linking physical inactivity with an increased risk of many adverse health conditions. Globally, physical inactivity is associated with 9% of premature mortality, meaning over five million deaths per year could be prevented. There is strong evidence linking physical inactivity with an increased risk of many adverse health conditions and, globally, physical inactivity is associated with 9% of premature mortality.13 For those with obesity and type 2 diabetes, studies of physical activity interventions have shown that early intervention can prevent or ameliorate weight gain and its health consequences, while also improving insulin sensitivity and reducing blood glucose levels (Table 1).14 For those on insulin, basal requirements can reduce as much as 30% after high-intensity interval exercise. As such, close glucose monitoring is required to make appropriate adjustments to treatment regimens when physical activity is planned.15
Although lifestyle interventions have demonstrated benefits in morbidity and mortality, the LookAHEAD trial highlights the complexity of the relationships among weight loss, glycaemic control and cardiovascular (CV) outcomes for individuals with type 2 diabetes.16 Despite achieving significant and sustained weight loss through caloric restriction and increased physical activity over an almost 10-year period trial participants did not have a statistically significant reduction in CV events. However, the intervention did lead to positive outcomes, including improved glycaemic control, blood pressure and lipid levels, as well as a higher rate of achieving and maintaining clinically significant weight loss. Some of these findings may relate to the unblinded nature of the study, in addition to the potential selection bias in the inclusion of more health-conscious patients. Regardless, these findings underscore the intricate interplay of factors in shaping CV outcomes within the context of lifestyle interventions for people with type 2 diabetes.
Very low energy diets
Although the sustained rate of type 2 diabetes remission in DiRECT was reassuring, there was a trend towards weight regain during the follow-up period. An analysis of eight high-quality weight loss studies showed that, without continued intervention, weight regain occurred in most people.17 Strategies that are easy to implement facilitate ongoing low caloric intake and a reduction in weight regain. Very low energy diets (VLEDs) are useful in reducing intake by providing 800 calories per day while ensuring the patient still has adequate intake of essential vitamins, minerals and amino acids. The low carbohydrate content of VLEDs induces a mild ketosis after two-to-three days. Despite their efficacy, the practical implementation of these diets necessitates careful consideration of individual circumstances and challenges; a substantial proportion of patients face difficulties adhering to and managing VLEDs. Reduced energy diets and low energy diets also exist as milder alternatives to VLEDs. Certain patients may be unsuitable for VLEDs, including those at risk of malnutrition or ketoacidosis (e.g. individuals prescribed a sodium-glucose cotransporter-2 [SGLT-2] inhibitor), individuals with a history of eating disorders and pregnant or lactating women. Patients undertaking VLEDs may experience fluctuations in glucose levels, necessitating careful monitoring and potential adjustments to medication.
Despite these considerations, the benefits of the anorexic effects of VLEDs make them a noteworthy consideration in managing type 2 diabetes and obesity. VLEDs should be considered as an initial weight loss strategy if supervised lifestyle interventions have been unsuccessful in reducing weight or if rapid weight loss is required (e.g. prior to bariatric or general surgery conditional on weight loss). Physical activity should be encouraged in those following a VLED, and VLEDs can be safely used in conjunction with other weight loss strategies. Intermittent use can also assist in the long-term management of obesity.
Pharmacotherapy
Although the above lifestyle strategies can help to lower body weight and induce type 2 diabetes remission, maintaining long-term weight loss with lifestyle interventions alone is difficult.18 Pharmacotherapy can assist patients to manage weight loss and weight maintenance. A significant barrier to ongoing pharmacotherapy use in Australia remains cost, as the long-term use of medications for weight loss is expensive and cost prohibitive for many people. Sadly, the management of obesity is strongly dictated by affordability.
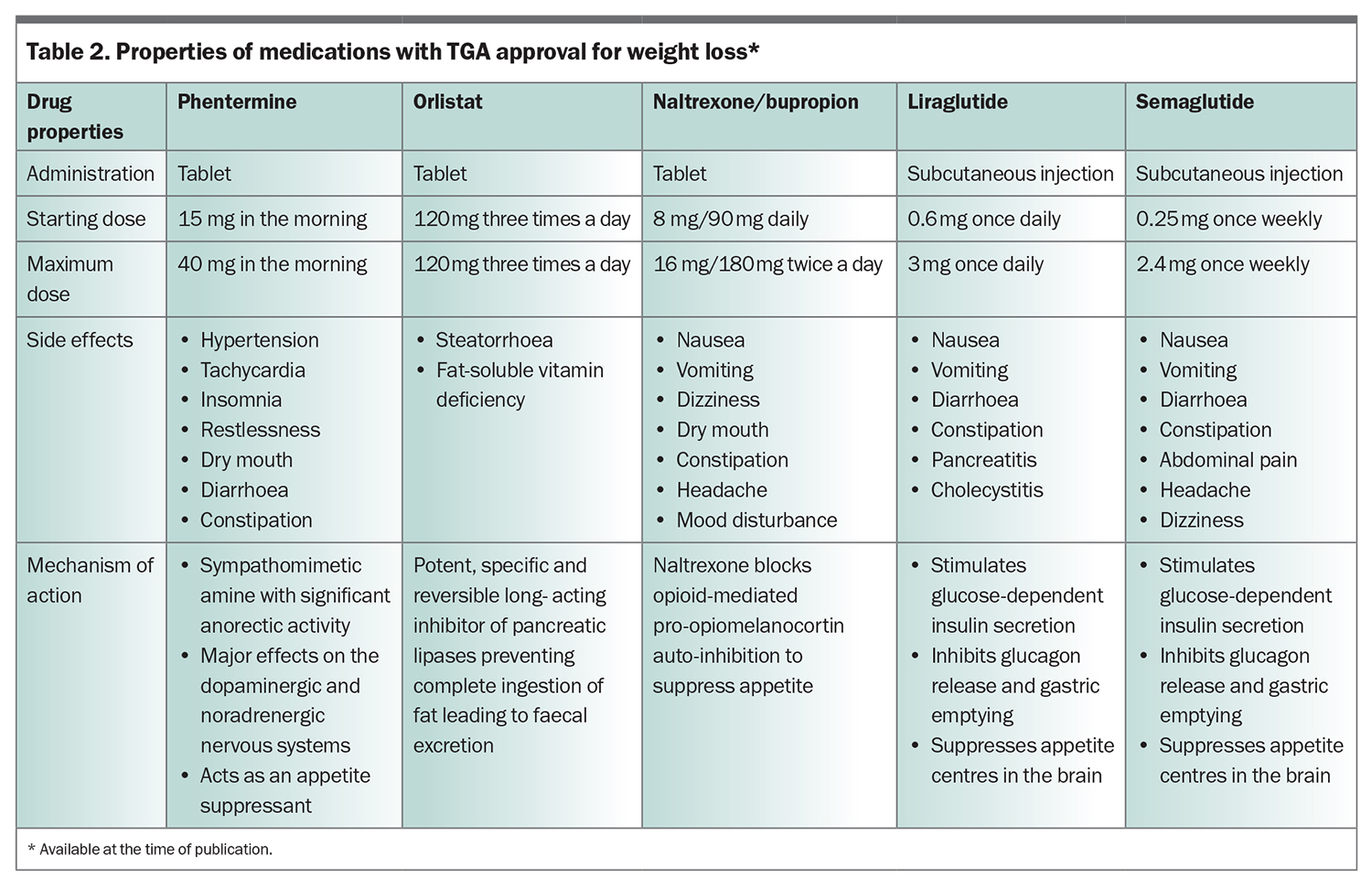
In Australia, the five antiobesity medications currently approved by the TGA for patients with obesity, or patients who are overweight with medical comorbidities are: orlistat, phentermine, combination naltrexone/bupropion, liraglutide and semaglutide (Table 2).19 Semaglutide is not currently recommended as a new prescription for patients in Australia, due to ongoing global shortages and supply issues.
Orlistat
Orlistat induces weight loss by inhibiting gastric and pancreatic lipases, which prevents the hydrolysis of triglycerides, thereby reducing the absorption of free fatty acids. Apart from its beneficial effects on weight, no direct glucose-lowering effects have been recognised with the use of orlistat.
Phentermine
Phentermine is an adrenergic agonist that increases the release of noradrenaline in the lateral hypothalamus. It is thought to induce weight loss through the inhibition of neuropeptide Y, which is involved in processes of hunger perception; however, the precise biochemical mechanism is not fully understood. As with orlistat, the use of phentermine has shown no direct glucose-lowering effects.
Naltrexone/bupropion
Although the exact neurochemical effects of naltrexone/bupropion are not fully understood, bupropion directly increases pro-opiomelanocortin (POMC) activity, and naltrexone indirectly increases POMC activity by blocking a natural negative feedback loop.20 Its direct administration into the brain in mice reduces food intake by altering the mesolimbic circuit in the brain and increases the firing rate of POMC neurons associated with regulation of appetite. In people with established type 2 diabetes, a 12-month trial of naltrexone/bupropion was found to reduce HbA1c by 0.6 mmol/mol in participants who had attained 5% body weight loss in the first 16 weeks and achieved a 9% body weight loss at 12 months.21
Liraglutide and semaglutide
The Australian Diabetes Society, Australian and New Zealand Obesity Society and Royal Australian College of General Practitioners recommend the early use of the glucagon-like peptide-1 receptor agonists (GLP-1RAs) liraglutide and semaglutide for the management of diabetes and obesity.22,23 The primary mechanism of action of GLP-1RAs involves the activation of the incretin pathway, which acts on the central nervous system to reduce appetite, resulting in reduced food intake and subsequent weight loss.24 Incretin-based therapy with GLP-1RAs stimulates insulin release from pancreatic beta cells and results in the reduction of body weight and blood glucose levels in people with type 2 diabetes.19 In patients with obesity, a 56-week treatment with liraglutide 3 mg resulted in a cumulative reduction in the risk of developing type 2 diabetes, as well as a mean body weight loss of 8.4 kg.25 In those with established prediabetes and obesity, liraglutide administered for three years led to a threefold increase in the likelihood of normoglycaemia.26 After a 30-week treatment course, liraglutide resulted in a reduction in HbA1c of 1.55 mmol/mol and mean body weight loss of 4.53 kg.27
Semaglutide 1 mg administered once-weekly by subcutaneous injection was found to lower body weight and sustain body weight reduction over a two-year trial period, with up to two-thirds of patients achieving a clinically meaningful reduction of at least 5% of their initial body weight.27 In addition to weight loss, semaglutide 1 mg is shown to have substantial potential in treating hyperglycaemia. Multiple head-to-head trials have shown its superior glucose-lowering efficacy compared with dipeptidyl peptidase-4 inhibitors, SGLT-2 inhibitors and other GLP-1RAs, with a mean HbA1c reduction of 1.5 to 1.8 mmol/mol and a HbA1c of less than 7 mmol/mol in 80% of patients.28-31 Additionally, multiple studies on GLP-1RAs have demonstrated reductions in CV risk, with up to 26% risk reduction in patients with high CV risk.27,32-34
In people with obesity and heart failure with preserved ejection fraction, semaglutide reduces heart failure symptoms and body weight and increases the duration of a six-minute walk test.35 Further, a trial of the effect of semaglutide on the progression of renal impairment in people with type 2 diabetes and chronic kidney disease (FLOW) was recently stopped early after an interim analysis revealed a positive outcome for semaglutide-treated participants. Results are expected in 2024 (ClinicalTrials.gov identifier: NCT03819153).
Tirzepatide
Tirzepatide, a new incretin-based therapy involving the activation of both the GLP-1 and gastric inhibitory polypeptide, has recently become available in Australia. Although it is currently only TGA approved for type 2 diabetes, it presents compelling prospects for the effective management of both obesity and diabetes. Compelling evidence also suggests a potential deceleration in the progression of chronic kidney disease.36 In the future, dual or even triple incretin-based therapy is expected to result in more potent weight loss effects. Notably, tirzepatide exhibited remarkable efficacy in a head-to-head comparison with the semaglutide 1 mg once-weekly regimen. Over a 40-week duration, the tirzepatide 15 mg once-weekly dosage resulted in an additional 0.45 mmol/mol reduction in HbA1c levels beyond semaglutide.37 Furthermore, the impact on weight dynamics was equally impressive, with tirzepatide 15 mg once-weekly yielding a mean percentage body weight loss of 20.9% after a comprehensive 72-week treatment course in people with obesity and without type 2 diabetes.38 Beyond glycaemic control and weight management, clinical trials have unveiled further advantages, including improvements in blood pressure, low-density lipoprotein cholesterol level and albuminuria.
Implications for weight management with other hypoglycaemic agents
Sodium-glucose contransporter-2 inhibitors
Although SGLT-2 inhibitors are less effective than GLP-1RAs in reducing body weight, they have an important role in providing substantial CV protection in patients with heart failure and diabetes. SGLT-2 inhibitors have been shown to reduce the risk of composite CV death or first hospitalisation for heart failure by 20%. Dapagliflozin and empagliflozin are PBS listed for adjunct therapy in patients diagnosed with heart failure (New York Heart Association class II, III or IV) with reduced ejection fraction (left ventricular ejection fraction <40%), even in the absence of diabetes.39 Empagliflozin is also PBS listed for adjunct therapy in those with heart failure with preserved ejection fraction, from 1 November 2023.
In patients diagnosed with renal impairment (with or without diabetes), dapagliflozin was shown to reduce the decline in estimated glomerular filtration rate, end-stage kidney disease and death from renal or CV causes by 39%.40 Dapagliflozin is PBS listed for proteinuric chronic kidney disease in addition to an ACE inhibitor or angiotensin receptor blocker.
Whether using an SGLT-2 inhibitor or a GLP-1RA, metformin should always be considered as first-line therapy to reduce insulin resistance. Although metformin is typically listed as a weight-neutral agent, it can promote weight loss in women with obesity and polycystic ovarian syndrome; however, it is not currently approved by the TGA for this indication.41
Insulin
Ideally, the management of type 2 diabetes should address the core metabolic derangements of the disease, namely insulin resistance and reduced insulin secretion. Beta cell loss is an intrinsic component of the pathogenesis of type 2 diabetes and, despite the increasing availability of alternative glucose- lowering medications, insulin may still be required to achieve adequate glycaemic control in people with type 2 diabetes (as a direct insulin injection or through increased insulin production, secondary to sulfonylureas). Although insulin can reduce the microvascular and macrovascular complications associated with poor glycaemic control, this is usually to the detriment of the patient’s body weight which generally increases. Insulin stimulates lipogenesis, inhibits protein catabolism and slows basal metabolism, thereby promoting fat mass gain. In combination with the abnormal peripheral administration route of insulin, these effects lead to reductions in energy metabolism and the well-known weight gain seen with insulin-dependent diabetes.42
Bariatric surgery
Weight-loss surgery is an important inclusion in the clinician’s armamentarium for the management of obesity and diabetes. Compared with other management strategies, weight-loss surgery is associated with long-term reductions in overall mortality, as well as decreased incidences of diabetes, myocardial infarction, stroke and cancer.43 Australian and New Zealand Obesity Society guidelines and Royal Australian College of General Practitioners guidelines recommend considering weight-loss surgery for people with a BMI greater than 35 kg/m2 and type 2 diabetes, or people with a BMI greater than 40 kg/m2 who are at high risk of developing type 2 diabetes when lifestyle interventions and medical therapy have been unsuccessful. The most common types of weight-loss surgery in Australia are gastric bypass (mini loop or Roux-en-Y) and sleeve gastrectomy.44 Although frequently performed in previous years, gastric banding is now uncommon due to its high failure rate and requirement for repeat procedures.
Many people with type 2 diabetes will experience improvement or normalisation of blood glucose levels after surgery, mandating close monitoring of medications in the postoperative period. These procedures do carry associated surgical and nutritional deficiency risks, with patients needing long-term monitoring of metabolic parameters, nutritional intake and bone health. Many patients who undergo weight-loss surgery do experience weight regain and remain at high risk of diabetes recurrence. With the advent of potent incretin-based therapies, it remains unclear whether pharmacotherapy should be mandated prior to consideration for surgery.
Conclusion
Diabetes and obesity are two of the highest contributors to the burden of chronic disease in Australia. A multipronged approach to tackling these interrelated conditions through weight loss can target the core metabolic derangements in both conditions and improve long-term morbidity and mortality. Lifestyle interventions paired with effective pharmacotherapy options have shown significant improvements in inducing weight loss and type 2 diabetes control. Such strategies should be considered early; ideally, before the development of comorbidities associated with type 2 diabetes and obesity. ET
COMPETING INTERESTS: Associate Professor Glastras has received honoraria and speaker fees, and taken part in advisory boards for Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi pharmaceutical companies. Dr Weir: None.
References
1. Australian Institute of Health and Welfare (AIHW). Diabetes. Canberra: AIHW; 2023. Available online at: https://www.aihw.gov.au/reports-data/health-conditions-disability-deaths/diabetes/overview (accessed October 2023).
2. Nianogo RA, Arah OA. Forecasting obesity and type 2 diabetes incidence and burden: the ViLA-obesity simulation model. Front Public Health. 2022; 10: 818816.
3. Overweight and obesity. Canberra: Australian Institute of Health and Welfare; 2020. Available online at: https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity/contents/summary (accessed Oct 2023).
4. Yuen M, Earle R, Kadambi N, et al. A systematic review and evaluation of current evidence reveals 195 obesity-associated disorders. New Orleans: The Obesity Society 2016.
5. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 Years. N Engl J Med 2017; 377: 13-27.
6. Whiteman DC, Webb PM, Green AC, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health 2015; 39: 477-484.
7. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9: 88.
8. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018; 391: 541-551.
9. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016; 22: 1-203.
10. Ryan D, Yockey S. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15% and over. Curr Obes Rep 2017; 6: 187-194.
11. Benraouane F, Litwin SE. Reductions in cardiovascular risk after bariatric surgery. Curr Opin Cardiol 2011; 26: 555-561.
12. Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation 2017; 135: 1577-1585.
13. Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012; 380: 219-229.
14. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract 2020; 49: 189-193.
15. Lee AS, Way KL, Johnson NA, Twigg SM. High-intensity interval exercise and hypoglycaemia minimisation in adults with type 1 diabetes: a randomised cross-over trial. J Diabetes Complications 2020; 34: 107514.
16. Pi-Sunyer X. The Look AHEAD Trial: a review and discussion of its outcomes. Curr Nutr Rep 2014; 3: 387-391.
17. Nordmo M, Danielsen YS, Nordmo M. The challenge of keeping it off, a descriptive systematic review of high-quality, follow-up studies of obesity treatments. Obes Rev 2020; 21: e12949.
18. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365: 1597-1604.
19. Pulipati VP, Pannain S. Pharmacotherapy of obesity in complex diseases. Clin Obes 2022; 12: e12497.
20. Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res 2014; 84: 1-11.
21. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes [published correction appears in Diabetes Care 2014; 37: 587]. Diabetes Care 2013; 36: 4022-4029.
22. Australian Type 2 Diabetes Glycaemic Management Algorithm. Sydney: Australian Diabetes Society; 2023. Available online at: https://www.diabetessociety.com.au/guideline/australian-type-2-diabetes-glycaemic-management-algorithm-august-2022/ (accessed Oct 2023).
23. Management of type 2 diabetes: A handbook for general practice. Melbourne: Royal Australian College of General Practitioners; 2022. Available online at: https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/diabetes/introduction (accessed Oct 2023).
24. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
25. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11-22.
26. Murphy CF, Docherty NG, le Roux CW. Liraglutide: another reason to target prediabetes? Oncotarget 2017; 8: 99203-99204.
27. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251-260.
28. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5: 341-354.
29. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 2018; 41: 258-266.
30. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6: 275-286.
31. Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 834-844.
32. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
33. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018; 392: 1519-1529.
34. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394: 121-130.
35. Kosiroborod M, Abildstrøm S, Borlaug B, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069-1084.
36. Bosch C, Carriazo S, Soler MJ, Ortiz A, Fernandez-Fernandez B. Tirzepatide and prevention of chronic kidney disease. Clin Kidney J 2022; 16: 797-808.
37. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
38. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205-216.
39. Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials [published correction appears in Lancet 2023;401: 104]. Lancet 2022; 400: 757-767.
40. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
41. Harborne LR, Sattar N, Norman JE, Fleming R. Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. J Clin Endocrinol Metab. 2005;90(8):4593-4598.
42. Type 1 diabetes - complications, pathogenesis, and alternative treatments [Internet]. Liu CP, ed. InTech; 2011. Available online at: http://dx.doi.org/10.5772/1540 (accessed Oct 2023).
43. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273: 219-234.
44. The Bariatric Surgery Registry 2022 Annual Report. Melbourne: Central Clinical School, Monash University; 2023. Available online at: https://www.monash.edu/__data/assets/pdf_file/0018/3351042/Bariatric-Surgery-Registry-Annual-Report-2022_web.pdf (accessed Oct 2023).