Obesity and cardiovascular disease: improving health outcomes

Obesity is a complex, multifactorial chronic disease that leads to the development of cardiovascular complications, both through its effect on traditional risk factors, including hypertension, dyslipidaemia and diabetes, and direct pathophysiological processes. Evidence shows that weight loss can reduce the occurrence of these risk factors and improve cardiovascular health outcomes in people with obesity.
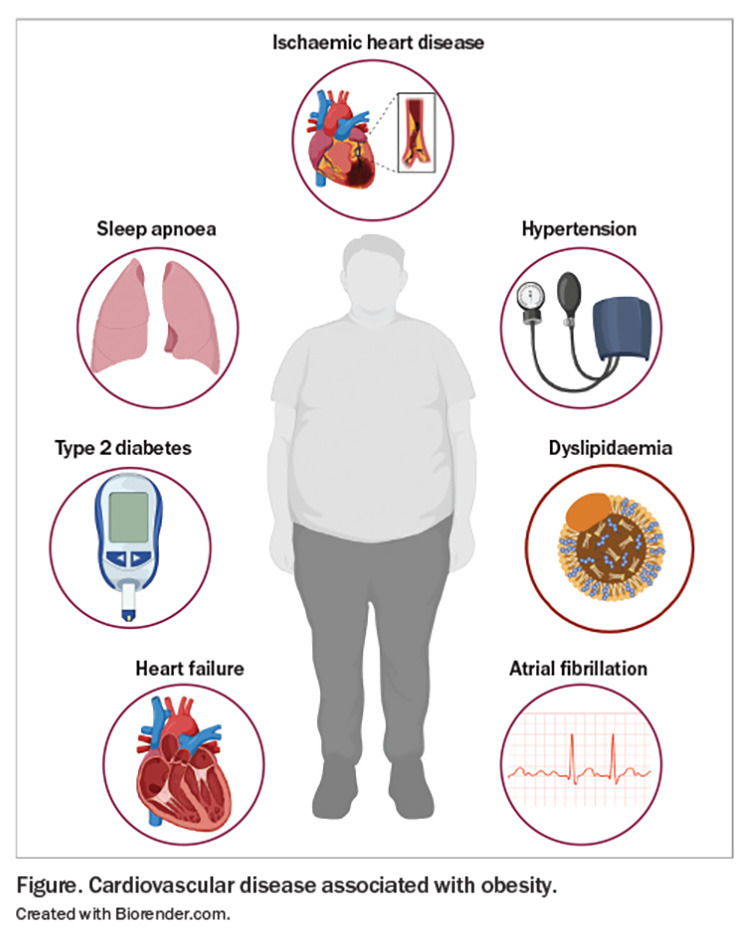
- Obesity contributes directly to risk factors for cardiovascular disease (CVD), including dyslipidaemia, type 2 diabetes, hypertension and obstructive sleep apnoea.
- Obesity is associated with the development of CVD and increased risk of CVD mortality, independent of cardiovascular risk factors.
- Abdominal obesity (and especially visceral adipose tissue), for which abdominal circumference is a surrogate marker, is an independent indicator of poor cardiovascular outcomes and increases the risk of all-cause mortality.
- Weight loss can improve associated risk factors and may reduce cardiovascular mortality.
Obesity is increasingly contributing to the burden of disease on a national and global level. Having tripled in prevalence since 1975, more than 1.9 billion adults worldwide were classified as being overweight or having obesity as of 2016.1 The Australian Institute of Health and Welfare revealed that in 2022, 66% of adults in Australia had an elevated body mass index (BMI), with 34% being overweight (BMI 25 to 29.9 kg/m2), 32% having obesity (BMI ≥30 kg/m2) and 13% of the total population having a BMI of 35 kg/m2 or higher.2 Indigenous Australians are 1.2 times more likely to be living with overweight or obesity and 1.5 times more likely to be living with obesity compared with the non-Indigenous population.2,3 High BMI is the second leading factor contributing to the health gap between Indigenous and non-Indigenous Australians.4
In addition to BMI, waist circumference may be used as an adjunct to the assessment of cardiovascular (CV) risk. Waist circumference has been shown to correlate with visceral adipose tissue (VAT) to a greater degree than BMI, an important point to consider given the nature of VAT as an independent risk marker for cardiovascular disease (CVD).5,6
Of the four million global deaths attributable to having a BMI of 25 kg/m2 or higher between 1985 and 2015, two-thirds were due to CVD.7 Given that CVD is the second most common cause of death among adults in Australia, the contribution of obesity to mortality is significant.8 An ‘obesity paradox’ exists, whereby people who are overweight or have obesity with established heart failure (HF), atrial fibrillation (AF), coronary artery disease (CAD) or hypertension have an improved prognosis for mortality compared with lean patients.9-12 These studies have largely been observational, epidemiological or retrospective. The data for both obesity as a risk factor for the development of CVD and for the beneficial effects of weight loss in the treatment of CVD are robust.13
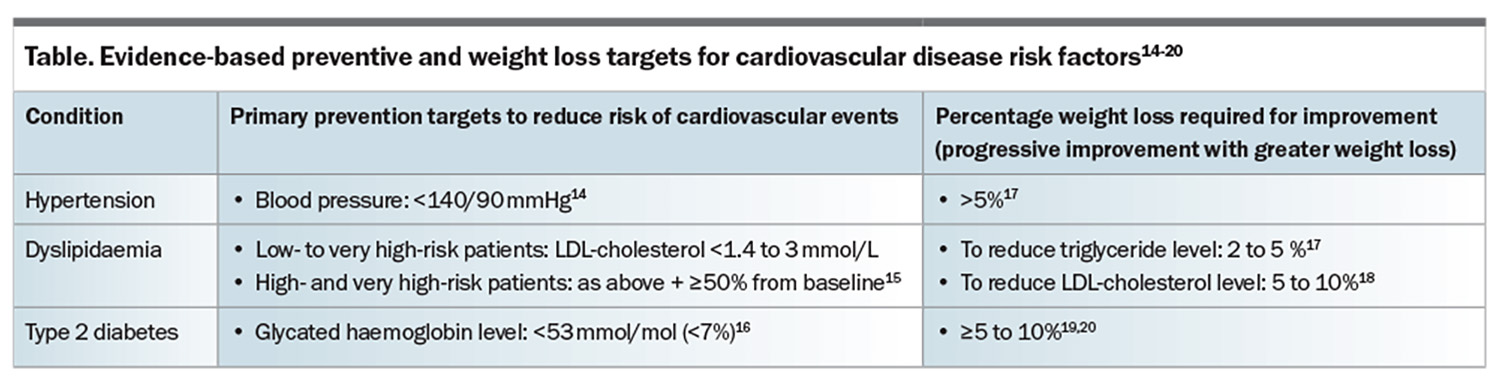
This article provides an overview of the association between obesity and CVD risk and outlines the evidence for the management of risk factors, particularly through weight loss, in reducing CV mortality and improving health outcomes. CVD associated with obesity is presented in the Figure, and primary prevention and weight loss targets for CVD risk factors are presented in the Table.14-20
Coronary artery disease
Obesity is a well-recognised independent risk factor for CAD. A meta-analysis of almost one million participants showed a 20% increase in the risk for CAD for each one standard deviation increase in BMI, independent of other risk factors.21 People with a greater number of excess BMI years are at higher risk of myocardial infarction (MI), hospitalisation for acute angina or acute coronary syndrome and coronary heart disease-related death. An excess BMI year is calculated as one year for each unit of BMI exceeding 25 kg/m2 cumulatively per year. For example, a person progressing from a BMI of 25 to 27 kg/m2 in the span of a year would have two excess BMI years. A person with 50 excess BMI years experiences CAD-related events at a rate 1.2 times greater than those without overweight or obesity.22
Central obesity and VAT have been linked to greater surface area involvement of coronary arterial lesions in young men.23 The presence of excess epicardial adipose tissue (EAT) has additionally been implicated in the onset of atherosclerotic CVD (ASCVD); people with obesity have increased thickness of EAT as visualised on transthoracic echocardiography.24 Increased EAT thickness leads to functional tissue changes, increased lipolysis-mediated release of fatty acids and subsequent local inflammation.25 The systemic inflammatory state of obesity and the shared microcirculation among EAT, the myocardium and coronary arteries may contribute to higher rates of ASCVD among people with obesity.25,26
Intentional weight loss of 5% through lifestyle (dietary and exercise) interventions in people with obesity and CAD disease has been shown to reduce all-cause and CV mortality, as well as major adverse CV events (relative risk, 0.67 over an average of 3.2 years’ follow up).27 Use of glucagon-like peptide-1 receptor agonists (GLP-1RAs) for the management of type 2 diabetes can reduce the occurrence of major adverse CV events.28-30 Even in people without diabetes who had pre-existing CVD and a BMI of 27 kg/m2 or higher, the GLP-1RA semaglutide 2.4 mg administered subcutaneously weekly was shown to reduce death from CV causes and nonfatal MIs and strokes by 20%, and led to a 10% mean reduction in body weight compared with placebo over a 40-month follow-up period.31 Patients with obesity who underwent bariatric surgery had a reduction in fatal and nonfatal MIs (mean weight loss, 16 to 23%; hazard ratio, 0.58) compared with those who received usual care or lifestyle modification.32
People with obesity have higher rates of hypertension, type 2 diabetes, chronic kidney disease and smoking, as well as being, on average, five years younger at time of presentation with CAD compared with people with a BMI less than 30 kg/m2.23,33
Hypertension
Obesity accounts for 65 to 78% of cases of essential hypertension.34 The prevalence of hypertension was shown to be 34% in people with a BMI below 25 kg/m2, and increased up to 74% in people with obesity.35 Increases in VAT deposition and subsequent altered adipokine levels (such as increases in leptin levels) can lead to hypertension through physical compression of the kidneys, activation of the renin-angio-tensinaldosterone system (RAAS) and mineralocorticoid receptors and increased renal sympathetic nerve activity.34 Increasing thickness of right ventricular EAT has also been associated with blunting of the normal reduction in blood pressure overnight, and increased rates of hypertension.36 These changes culminate in increased left ventricular pressure and volume overload, and subsequent macrophage-induced myocyte hypertrophy and dysfunction.37,38 A 22% greater reduction in the risk of hypertension occurred in a cohort of patients with an initial BMI of 40 kg/m2 who achieved a mean weight loss of 13% over a six-year period, compared with patients with an initial and stable BMI of 30 kg/m2.39
Weight loss of at least 5% in those with overweight or obesity can reduce blood pressure.17 With respect to primary prevention, pharmacological treatment for hypertension should be considered in patients aged 18 to 79 years with a systolic blood pressure of 140 mmHg or higher, or diastolic blood pressure of 90 mmHg or higher to reduce the risk of fatal and nonfatal CV events.14
Dyslipidaemia
Evidence of dyslipidaemia is seen in 50 to 70% of people with obesity,40 and comprises elevated triglyceride, LDL-cholesterol, very low-density lipoprotein-cholesterol and apolipoprotein-B levels and decreased HDL-cholesterol levels.41-43 There is a strong body of evidence behind treating elevated LDL-cholesterol for CV reduction. In people with overweight or obesity, weight loss in the range of 5 to 10% with lifestyle interventions and use of older-generation obesity medications may lead to a 0.20 mmol/L reduction in LDL-cholesterol.18 The use of statin therapy was shown to reduce the risk of major vascular events, determined as major coronary events, nonfatal or fatal strokes and coronary revascularisation, by 23% per 1 mmol/L reduction in LDL-cholesterol; all-cause mortality was reduced by 12% per 1 mmol/L reduction in LDL-cholesterol compared with no statin therapy.44 Hypertriglyceridaemia is independently associated with an increased risk of CV events.45 Weight loss of 2 to 5% was shown to reduce triglyceride levels, which progressively decreased with greater weight loss;17 however, there is limited evidence to support the routine addition of fibrates to reduce CVD risk in patients already on statin therapy.47
There are currently no guidelines for lipid management specifically in people with obesity – rather, guidelines recommend management depending on total CV risk based on multiple risk factors. The European Society of Cardiology 2019 guidelines for the management of dyslipidaemias recommend that a graded approach be utilised, with LDL-cholesterol targets of:15
- less than 3.0 mmol/L for low-risk patients
- less than 2.6 mmol/L for moderate-risk patients
- less than 1.8 mmol/L for high-risk patients
- less than 1.4 mmol/L for very high-risk patients.
Additionally, a 50% or greater reduction in LDL-cholesterol levels from baseline is recommended for high- and very high-risk patients.15
Type 2 diabetes
In 2017–18, adults with obesity in Australia were almost five times more likely to develop type 2 diabetes compared with people without obesity.48 Excessive adipose tissue, particularly in a central/visceral distribution, increases the risk of insulin resistance, whereas gluteofemoral adipose tissue is protective against declining beta-cell function.49,50 The mechanisms by which obesity increases the risk of diabetes are not fully understood, but involve both adiposity-induced impairment of beta-cell function and multiorgan insulin resistance.51
CVD is responsible for over 50% of deaths in patients with type 2 diabetes.52 Even in people without diabetes, insulin resistance is associated with a 1.4-fold increase in CV events.53 These increased risks also translate to in-hospital mortality: patients with type 2 diabetes have a 15% higher risk of MI-related mortality and 6% higher risk of mortality related to a composite of CVD during admission compared with matched controls without diabetes.54
Targets for glycaemic control vary based on individual patient factors; however in general, a glycated haemoglobin target below 53 mmol/mol (<7%) is recommended.16 If clinically appropriate, weight loss should be prioritised alongside glycaemic control and cardiorenal protection in patients with type 2 diabetes.55 Weight loss of 10 to 15% can lead to remission of diabetes in most patients with a short duration (<6 years) of type 2 diabetes.20 Even if large weight loss or diabetes remission is not appropriate or achievable, a reduction in body weight of 5 to 10% is likely to improve metabolic control and risk factors for cardiometabolic disease.19
Heart failure
Total HF risk for both HF with preserved ejection fraction (HFpEF), where the left ventricular ejection fraction (LVEF) is 50% or above, and reduced ejection fraction (HFrEF), where the LVEF is 40% or below, increases by 5% in men and 7% in women for each 1 kg/m2 increase in BMI.56 The chronic inflammatory state of obesity (compounded by hypertension and type 2 diabetes) causes left ventricular hypertrophy, microvascular dysfunction and myocardial fibrosis. The risk of developing HFpEF increases as BMI increases.57 Activation of the sympathetic nervous system as well as the RAAS through increased adipokine synthesis and mechanical renal compression leads to increased total blood volume and increased rates of both left ventricular concentric remodelling and right ventricular wall stiffness.58,59 Increased leptin production has also been shown to lead to cardiomyocyte hypertrophy and fibrosis via activation of angiotensin-2, mineralocorticoid and endothelin-1 receptors.60 Systemic inflammatory changes arise through an imbalance between pro- and anti-inflammatory cytokines, which then leads to reduced capillary density and mitochondrial dysfunction and downregulation of coronary vasodilation within cardiac muscle.61 EAT contributes to the inflammatory state, as it can act in a paracrine manner, releasing proinflammatory adipocytokines, and also functions as a ‘space-occupying lesion’, leading to enhanced pericardial restraint and interventricular dependence.58
There is evidence of increased HF-related mortality in people with obesity and HFpEF. Patients with HFpEF and increased abdominal adiposity are at higher risk of all-cause, CV and nonCV mortality compared with patients without excess abdominal adiposity, as measured by waist circumference.62 Weight loss via caloric restriction or aerobic exercise, or both, was shown to increase exercise capacity, as measured by VO2 max. The greatest effects on weight loss were seen with combined diet and exercise, leading to a 10% decrease in body weight, compared with a 7% decrease with diet and 3% decrease with exercise alone.63 In people with HFpEF and a BMI above 30 kg/m2, with and without type 2 diabetes, use of semaglutide 2.4 mg given weekly for one year improved HF-related symptoms and achieved a 6 to 11% higher mean weight loss compared with placebo.64,65
There is evidence for the benefit of intentional weight loss for several HFrEF outcomes, including mortality.66 The New York Heart Association (NYHA) classification provides a guide to symptomatic burden related to heart failure on a scale of 1 (no symptoms) to 4 (symptoms at rest). A recent meta-analysis showed that intentional weight loss in patients with HFrEF or HFpEF and obesity may lead to improved exercise tolerance, NYHA classification and quality of life.66 Furthermore, two recent systematic reviews showed improvement in cardiac structure and function, including LVEF and NYHA classification, in patients with either HFpEF or HFrEF following bariatric metabolic surgery.67,68
Obstructive sleep apnoea
Obesity is a major risk factor for obstructive sleep apnoea (OSA). Rates of OSA in the general population are reported between 9 and 38%, depending on cohort characteristics, and increase with increasing BMI.69 In an epidemiological study, OSA was identified in 85% of men and 56% of women with obesity compared with 29% of men and 11% of women with a BMI below 25 kg/m2.70 Compared with stable weight, a 10% increase in weight was associated with a 32% increase in the apnoea-hypopnoea index.71 The risk of OSA increases because of decreased pharyngeal airway lumen and increased risk of pharyngeal collapse.72 The reduction in functional residual capacity compared with residual volume may further compound this risk, which may further reduce lung volume and stability of respiratory control, especially when supine.73 Other contributing pathophysiological changes include decreased muscle activity, increased upper airway resistance and modest increases in arterial carbon dioxide level.74 Large negative pressure swings seen in patients with OSA ultimately lead to increased left ventricular oxygen demand, sympathetic activity and a heightened pro-arrhythmic state.74
OSA is a risk factor for sudden cardiac death, AF and systemic hypertension.74-76 It is present in 20 to 55% of patients across a range of HF syndromes, including HFrEF and HFpEF.77 Intermittent hypoxia leads to the accelerated development of atherosclerosis and vascular remodelling via proinflammatory states and increased myocardial sensitivity to ischaemia and reperfusion.78-80 Although there is no strong evidence to support the use of positive-airway pressure (PAP) treatment to reduce the risk of CVD-related mortality,81 there is strong evidence for its use in managing hypertension in people with OSA.82 PAP also showed modest improvements in systolic function in those with HFrEF; however, there is no consistent evidence to suggest that it reduced the risk of CAD in this cohort.83
Atrial fibrillation
About one in five cases of AF are attributable to obesity, with several obesity-related factors contributing to the epidemic of AF.86 Obesity- related risk factors, such as hypertension, diabetes and OSA, as well as proinflammatory and profibrotic factors, cause downstream structural and electrical remodelling, which contributes to an increased risk of AF.86 EAT has also been implicated in the pathogenesis of AF in patients with obesity via proinflammatory mediators, as well as autonomic nervous system stimulation secondary to the presence of ganglionated plexi within EAT.86 The rates of progression of paroxysmal HF to its persistent subtype are increased in people with obesity.87 Elevated BMI also adversely affects ablation outcomes, with patients who are overweight or have obesity experiencing higher rates of AF recurrence following ablation compared with those with a normal BMI.88 Increased rates of minor complications have been shown to occur in patients who have obesity, typically due to an increased duration of hospital stay secondary to fluid overload.89 Some studies have shown a threefold increased risk of major complications in patients with severe obesity.90
Weight loss can reduce AF symptom burden and severity in patients with obesity. Weight loss in patients receiving weight management intervention was significantly higher and associated with improved AF symptom burden and severity and a decline in AF duration compared with patients receiving general lifestyle advice.91 Sustained weight loss was associated with a significant reduction in AF burden; patients who maintained a weight loss of 10% or higher over four years had a sixfold greater likelihood of arrhythmia-free survival compared with those with weight loss of less than 10%.92 Weight loss greater than 10% among patients with obesity was shown to mitigate the progression of paroxysmal to permanent AF and was associated with reversion of paroxysmal AF to sinus rhythm.93
Conclusion
Obesity increases the risk of CVD both directly and indirectly. GPs have a key role in the primary prevention of CVD, and in the identification and management of patients living with obesity and CVD. Weight loss improves CV health through direct risk reduction, better management of underlying risk factors and reduced rates of complications. Weight loss in the range of 10% or higher confers overall reduced rates of CV morbidity and mortality and improved quality of life. ET
COMPETING INTERESTS: Dr Bradbury and Dr Gutman: None. Associate Professor Sumithran has coauthored manuscripts with writing assistance provided by Novo Nordisk and Eli Lilly.
References
1. World Health Organization (WHO). Obesity and overweight. March 2024. Geneva; WHO, 2024. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed July 2024).
2. Australian Institute of Health and Welfare (AIHW). Overweight and obesity. June 2024. Canberra; AIHW, 2024. Available online at: https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity (accessed July 2024).
3. Australian Bureau of Statistics (ABS). National Aboriginal and Torres Strait Islander Health Survey Methodology. 2018-19 financial year. Canberra; ABS, 2019. Available online at: https://www.abs.gov.au/methodologies/national-aboriginal-and-torres-strait-islander-health-survey-methodology/2018-19 (accessed July 2024).
4. Al-Yaman F. The Australian Burden of Disease Study: impact and causes of illness and death in Aboriginal and Torres Strait Islander people, 2011. Public Health Res Pract 2017; 27: 2741732.
5. Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7: 715-725.
6. Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care 2018; 21: 360-365.
7. GBD 2015 Obesity Collaborators; Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13-27.
8. Australian Bureau of Statistics (ABS). Provisional mortality statistics. Canberra; ABS, 2024. Available online at: https://www.abs.gov.au/statistics/health/causes-death/provisional-mortality-statistics/latest-release (accessed July 2024).
9. Sharma A, Lavie CJ, Borer JS, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015; 115: 1428-1434.
10. Sandhu RK, Ezekowitz J, Andersson U, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J 2016; 37: 2869-2878.
11. Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol 2014; 29: 801-812.
12. Jayedi A, Shab-Bidar S. Nonlinear dose-response association between body mass index and risk of all-cause and cardiovascular mortality in patients with hypertension: a meta-analysis. Obes Res Clin Pract 2018; 12: 16-28.
13. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009; 53: 1925-1932.
14. Mancia G, Kreutz R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens 2023; 41: 1874-2071.
15. Mach F, Baigent C, Catapano AL, et al; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111-188.
16. American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022; 45(Suppl 1): S83-S96.
17. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481-1486.
18. Zomer E, Gurusamy K, Leach R, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes. Rev 2016; 17: 1001-1011.
19. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet 2022; 399: 394-405.
20. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018; 391: 541-551.
21. Riaz H, Khan MS, Siddiqi TJ, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open 2018; 1: e183788.
22. Reis JP, Allen N, Gunderson EP, et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity 2015; 23: 879-885.
23. McGill HC Jr, McMahan CA, Herderick EE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002; 105: 2712-2718.
24. Aitken-Buck HM, Moharram M, Babakr AA, et al. Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte 2019; 8: 412-420.
25. Packer, M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018; 71: 2360-2372.
26. Ghigliotti G, Barisione C, Garibaldi S, et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 2014; 37: 1337-1353.
27. Pack QR, Rodriguez-Escudero JP, Thomas RJ, et al. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc 2014; 89: 1368-1377.
28. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
29. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394: 121-130.
30. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
31. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al; SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221-2232.
32. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307: 56-65.
33. Velazquez G, Gomez TMA, Asemota I, et al. Obesity impacts mortality and rate of revascularizations among patients with acute myocardial infarction: an analysis of the national inpatient sample. Cureus 2020; 12: e11910.
34. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015; 116: 991-1006.
35. Bramlage P, Pittrow D, Wittchen HU, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 2004; 17: 904-910.
36. Guan B, Liu L, Li X, et al. Association between epicardial adipose tissue and blood pressure: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2021; 31: 2547-2556.
37. da Silva AA, do Carmo JM, Wang Z, Hall JE. Melanocortin-4 receptors and sympathetic nervous system activation in hypertension. Curr Hypertens Rep 2019; 21: 46.
38. Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res 2020; 126: 789-806.
39. Haase CL, Lopes S, Olsen AH, et al. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes 2021; 45: 1249-1258.
40. Dias S, Paredes S, Ribeiro L. Drugs involved in dyslipidemia and obesity treatment: focus on adipose tissue. Int J Endocrinol 2018; 2018: 2637418.
41. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med 2015; 373: 1307-1317.
42. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013; 5: 1218-1240.
43. Feingold KR. Obesity and Dyslipidemia. 2023 Jun 19. In: Feingold KR, Anawalt B, Blackman MR, et al. Eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 26247088.
44. Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267-1278.
45. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011; 123: 2292-2333.
46. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366: 1849-1861.
47. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1563-1574.
48. Australian Bureau of Statistics (ABS). Diabetes. 2017-18 financial year. Canberra; ABS, 2018. Available online at: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/diabetes/2017-18 (accessed July 2024).
49. Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology 2002; 123: 882-932.
50. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010; 34: 949-959.
51. Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab 2022; 34: 11-20.
52. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018; 17: 83.
53. Robins SJ, Rubins HB, Faas FH, et al; Veterans Affairs HDL Intervention Trial (VA-HIT). Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care 2003; 26: 1513-1517.
54. de Miguel-Yanes JM, Jiménez-García R, Hernández-Barrera V, Méndez-Bailón M, de Miguel-Díez J, Lopez-de-Andrés A. Impact of type 2 diabetes mellitus on in-hospital-mortality after major cardiovascular events in Spain (2002-2014). Cardiovasc Diabetol 2017; 16: 126.
55. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022; 45: 2753-2786.
56. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305-313.
57. Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure. Circ Heart Fail 2013; 6: 279-286.
58. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136: 6-19.
59. Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res 2023; 118: 3434-3450.
60. Huby AC, Antonova G, Groenendyk J, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015; 132: 2134-2145.
61. Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res 2021; 117: 423-434.
62. Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 2017; 70: 2739-2749.
63. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016; 315: 36-46.
64. Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al; STEP-HFpEF Trial Committees and Investigators. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069-1084.
65. Kosiborod MN, Petrie MC, Borlaug BA, et al; STEP-HFpEF DM Trial Committees and Investigators. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med 2024; 390: 1394-1407.
66. McDowell K, Petrie MC, Raihan NA, Logue J. Effects of intentional weight loss in patients with obesity and heart failure: a systematic review. Obes Rev 2018; 19: 1189-1204.
67. Esparham A, Mehri A, Hadian H, et al. The effect of bariatric surgery on patients with heart failure: a systematic review and meta-analysis. Obes Surg 2023; 33: 4125-4136.
68. Sargsyan N, Chen JY, Aggarwal R, Fadel MG, Fehervari M, Ashrafian H. The effects of bariatric surgery on cardiac function: a systematic review and meta-analysis. Int J Obes (Lond) 2024; 48: 166-176.
69. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017; 34: 70-81.
70. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010; 11: 441-446.
71. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000; 284: 3015-3021.
72. Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol (1985) 2010; 108: 430-435.
73. Malhotra A, Hillman D. Obesity and the lung: 3. Obesity, respiration and intensive care. Thorax 2008; 63: 925-931.
74. Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. Am Coll Cardiol 2017; 69: 841-858.
75. Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62: 610-616.
76. Li Y, Miao Y, Zhang Q. Causal associations of obstructive sleep apnea with cardiovascular disease: a Mendelian randomization study. Sleep 2023; 46(3): zsac298.
77. Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021; 144: e56-e67.
78. Arnaud C, Bochaton T, Pépin JL, Belaidi E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch Cardiovasc Dis 2020; 113: 350-358.
79. Belaidi E, Thomas A, Bourdier G, et al. Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int J Cardiol 2016; 210: 45-53.
80. Baguet JP, Boutin I, Barone-Rochette G, et al. Hypertension diagnosis in obstructive sleep apnea: self or 24-hour ambulatory blood pressure monitoring? Int J Cardiol. 2013; 167: 2346-2347.
81. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375: 919-931.
82. Feldstein CA. Blood pressure effects of CPAP in nonresistant and resistant hypertension associated with OSA: a systematic review of randomized clinical trials. Clin Exp Hypertens 2016; 38: 337-346.
83. Chang JL, Goldberg AN, Alt JA, et al. International consensus statement on obstructive sleep apnea. Int Forum Allergy Rhinol 2023; 13: 1061-1482.
84. Carneiro-Barrera A, Amaro-Gahete FJ, Guillén-Riquelme A, et al. Effect of an interdisciplinary weight loss and lifestyle intervention on obstructive sleep apnea severity: the INTERAPNEA Randomized Clinical Trial. JAMA Netw Open 2022; 5: e228212.
85. Foster GD, Borradaile KE, Sanders MH, et al; Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169: 1619-1626.
86. Sha R, Baines O, Hayes A, et al. Impact of obesity on atrial fibrillation pathogenesis and treatment options. J Am Heart Assoc 2024; 13: e032277.
87. Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc 2014; 3: e000916.
88. Mangiafico V, Saberwal B, Lavalle C, et al. Impact of obesity on atrial fibrillation ablation. Arch Cardiovasc Dis 2020; 113: 551-563.
89. Winkle RA, Mead RH, Engel G, et al. Impact of obesity on atrial fibrillation ablation: Patient characteristics, long-term outcomes, and complications. Heart Rhythm 2017; 14: 819-827.
90. Shoemaker MB, Muhammad R, Farrell M, et al. Relation of morbid obesity and female gender to risk of procedural complications in patients undergoing atrial fibrillation ablation. Am J Cardiol 2013; 111: 368-373.
91. Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013; 310: 2050-2060.
92. Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015; 65: 2159-2169.
93. Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018; 20: 1929-1935.