Osteoporosis in men: underestimated, underdiagnosed and undertreated

Osteoporosis in men is a common yet often overlooked condition, frequently remaining undiagnosed and untreated. Despite having higher bone mass and lower rates of lifetime fractures compared with women, men experience higher rates of post-fracture mortality and are more likely to have a secondary cause of osteoporosis. Bone mineral density should be assessed in men aged 70 years and older and those with a previous fracture. Optimal management includes preventive interventions and pharmacological therapies.
- One in six men will sustain a fracture by the age of 90 years, with almost half occurring before age 80.
- One-third of all hip fractures occur in men, with a mortality rate of up to 38% in the first year after a fracture.
- Most nonvertebral fractures occur in men whose bone mineral density is not in the osteoporotic range.
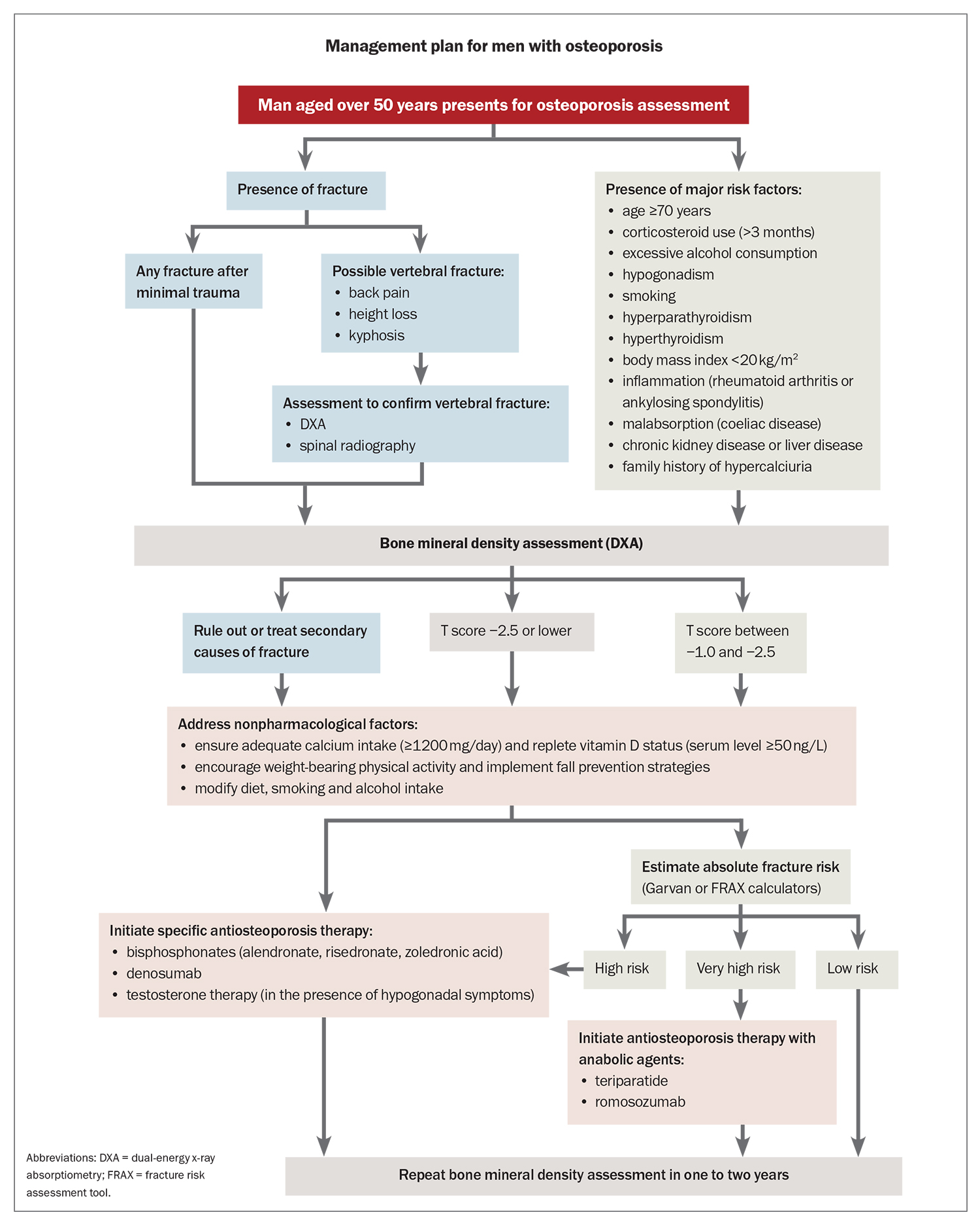
- All men aged 70 years or older, as well as those with a minimal trauma fracture, should undergo assessment with a bone mineral density scan and be investigated for common secondary causes.
- Regular weight-bearing exercise, adequate calcium intake, optimal vitamin D status and avoidance of smoking and excessive alcohol consumption are important preventative measures.
- Oral bisphosphonates, six-monthly subcutaneous denosumab, or annual intravenous zoledronic acid are recommended as first-line medical therapies.
- Men at the highest or imminent risk of fracture should be referred to a specialist for consideration of anabolic therapy.
Osteoporosis remains an underestimated, underdiagnosed and undertreated public health issue in men. Globally, one-third of all hip fractures occur in men, with almost half occurring before the age of 80 years.1,2 Furthermore, one in six men will sustain a fracture by the age of 90, highlighting the need for its earlier diagnosis and management.3
Men older than 60 years of age face an eightfold increase in mortality risk during the first three months following a hip fracture.4 Moreover, compared with women, more men die within the first year of experiencing a hip fracture, with a mortality rate of up to 38%.5 Up to 40% of hip fractures occur among men in residential care facilities, and 20% of men who fracture their hip will experience a subsequent hip fracture.
Vertebral fractures are also common among elderly men and, although most are painless, may lead to height loss, reduced quality of life, respiratory dysfunction, and increased mortality and risk of subsequent hip and other fractures.6 In men, vertebral fractures peak during the fourth and fifth decades of life and may be associated with trauma; however, fracture rates decline with age, reaching only half the rate compared with women.7-9
Given that most nonvertebral fractures occur in men with bone mineral density (BMD) not in the osteoporotic range, it is imperative to consider clinical risk factors other than BMD in determining absolute fracture risk in men. This article describes the current evidence on, and presents the optimal approach to, screening, diagnosis and management of osteoporosis in men.
Natural history of bone loss in men
Peak bone mass is influenced by genetic and environmental factors, and is achieved by the age of 20 years at the hip and by 30 years at the spine for both men and women.10,11 However, men have a 30% larger bone cross-sectional area and 20% higher bone mass compared with women, which is consistent with their larger skeletal size. These differences between men and women are believed to be due to the effects of increased androgen concentrations and a longer bone maturation period in men.12,13
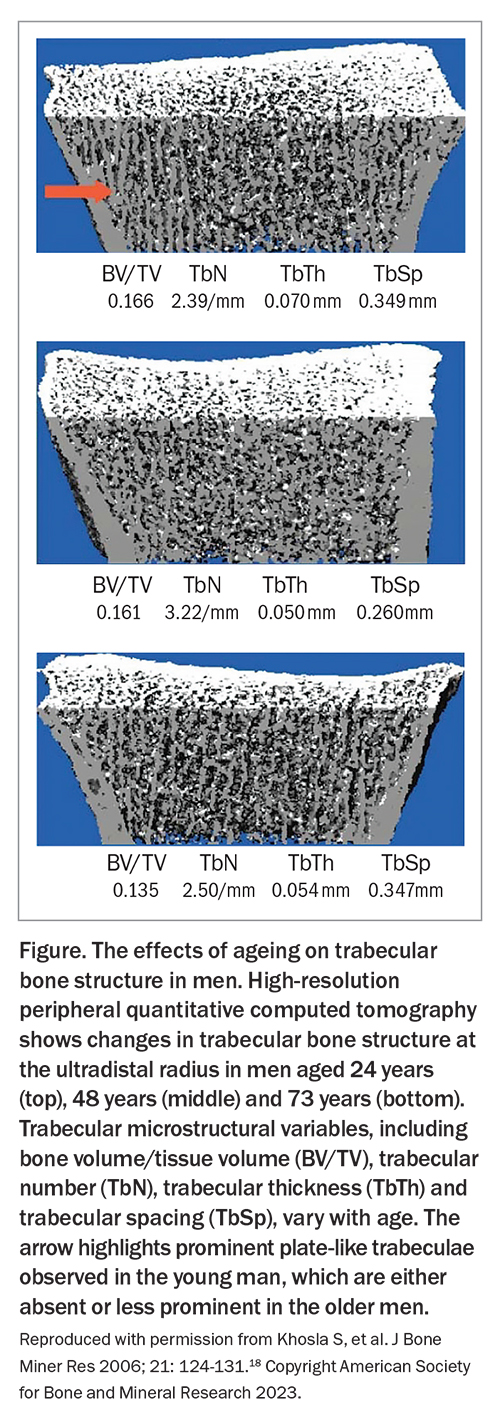
Bone loss begins shortly after reaching peak bone mass. Longitudinal studies suggest that bone loss accelerates after the age of 70 years in men, particularly in those with deficient serum testosterone or oestradiol levels.14-16 Trabecular bone loss starts early, with up to 42% of total lifetime trabecular bone loss occurring before the age of 50 years, possibly due to changes in insulin-like growth factor 1 concentration. In men, reduced bone formation results in loss of trabecular thickness, whereas women experience reduced trabecular number due to increased bone resorption (Figure).17,18 The preservation of trabecular number seen in men may contribute to their favourable lifetime fracture profile when compared with women.19 Cortical bone loss in men occurs later in life, with 85% occurring after the age of 50 years, accompanied by reductions in bioavailable sex steroids and increased bone remodelling.17
Secondary causes of bone loss in men
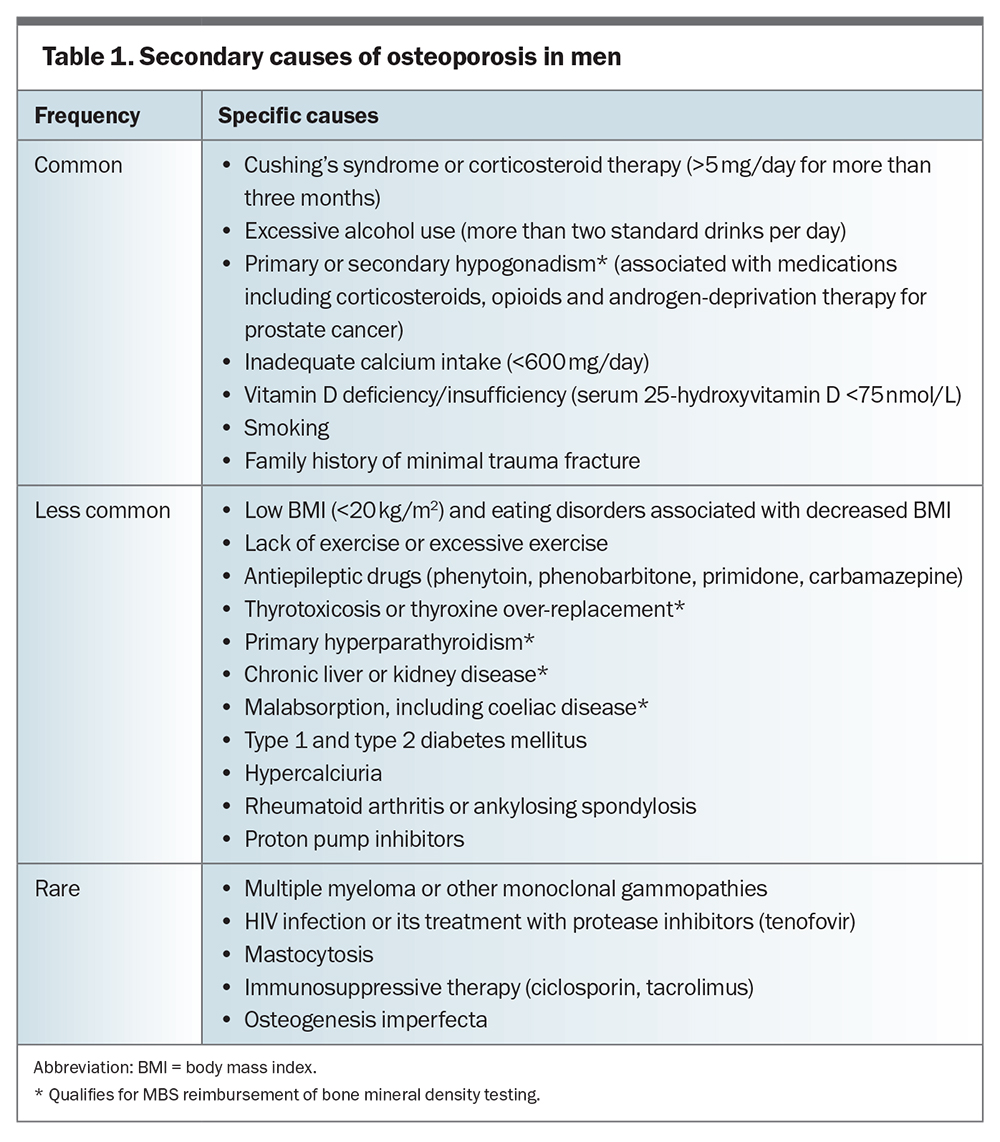
Secondary causes of osteoporosis are more prevalent in men than in postmenopausal women and should be taken into consideration (Table 1). The most common secondary causes, accounting for most cases, include corticosteroid use, excessive alcohol consumption, smoking and hypogonadism. Identifying causes of secondary hypogonadism, such as from androgen deprivation therapy in prostate cancer, is particularly important as significant bone loss may occur as early as six months after initiating therapy.20 Vitamin D deficiency is a less common cause and is associated with an increased risk of hip fracture in both men and women aged over 65 years when serum levels of 25-hydroxyvitamin D are below 63 nmol/L.21
Screening and diagnosis
Dual-energy x-ray absorptiometry (DXA) is a robust predictor of fractures and is used to measure BMD. Although quantitative CT can also estimate BMD, it should not be used as a first-line investigation as it is not diagnostically validated and has higher radiation exposure and costs than DXA.22 The relationship between BMD and fracture risk is continuous, with each standard deviation (SD) decrease in hip BMD associated with a 2.6-fold increase in relative risk of hip fracture.23
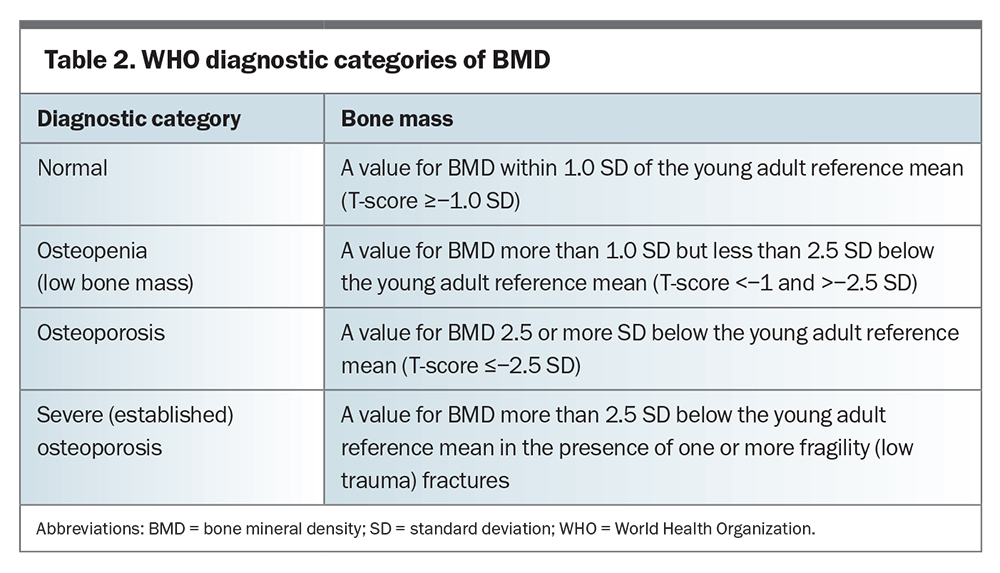
The WHO has established diagnostic thresholds for osteoporosis and osteopenia based on total-hip BMD in postmenopausal women, which are also applied to men and at other anatomical sites (Table 2). For any given absolute BMD value at the spine or hip, the risk of fracture is similar between men and women of the same age.24-26 However, the average BMD in men who experience hip fractures is higher than that in women, potentially due to factors such as deterioration in bone microarchitecture and trauma, which are not reflected in BMD values. Using female data ranges for BMD to calculate T-scores in men significantly reduces the prevalence of men classed as having osteoporosis and osteopenia.27 Therefore, a sex-specific T-score (the number of SDs from the normal young mean bone density) based on a male normative database is used to address this discrepancy. However, factors other than BMD alone need to be considered to accurately determine fracture risk in men.
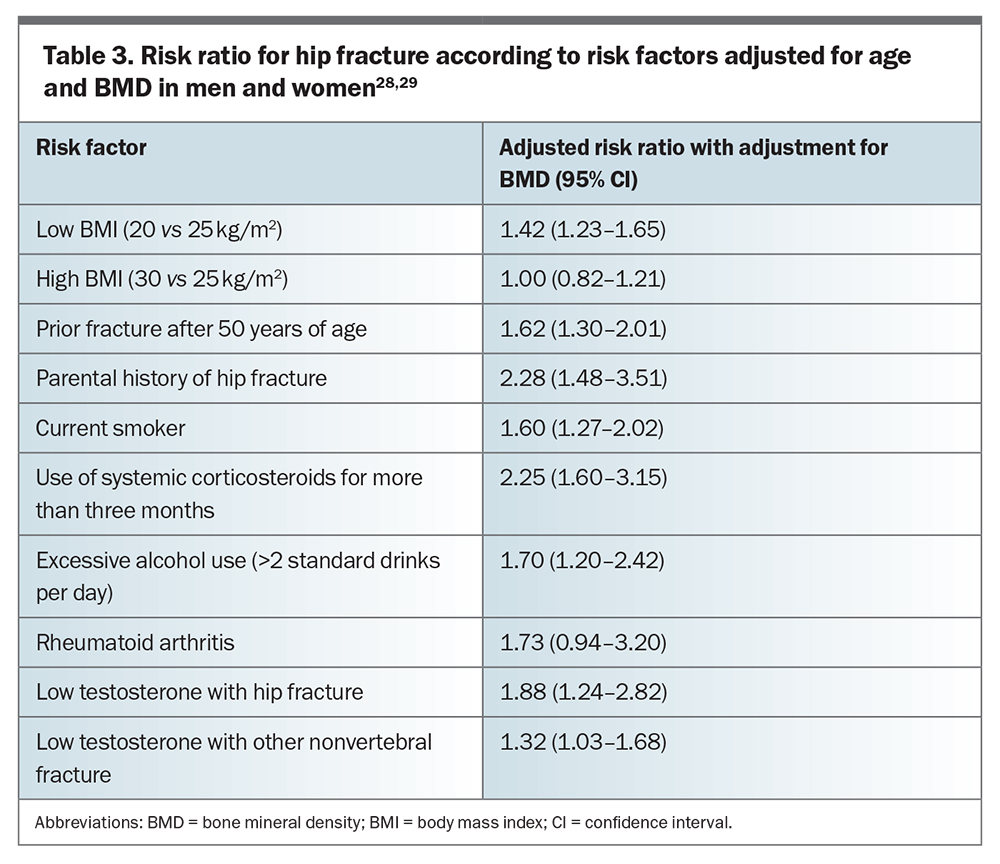
DXA is recommended for all men aged 70 years and older, and earlier for men with major clinical risk factors for osteoporosis. BMD measurements at the femoral neck and total hip are preferable to measurements at the spine or radius. Men should be routinely assessed for clinical signs of secondary causes of, and other risk factors for, osteoporosis (Table 3).28,29 A history of minimal trauma fracture after the age of 50 years is the strongest clinical risk factor for fracture.30 Examination findings, such as a loss of height or an occiput to wall distance of more than 2 cm, may be suggestive of an underlying vertebral fracture. Unfortunately, spinal fractures are often asymptomatic and are best detected using a spinal x-ray, which will show a significant loss of vertebral height (≥20%). Lesser deformities may be attributed to spinal degenerative changes or congenital anomalies (e.g. Schmorl’s nodes in Scheuermann’s disease). Lateral spine images obtained during DXA scans can also be used to screen for vertebral fractures.
The calculation of absolute fracture risk over five or 10 years can be performed using either the Fracture Risk Assessment (FRAX) tool (www.sheffield.ac.uk/FRAX/tool.aspx) or the Garvan Fracture Risk Calculator (https://www.garvan.org.au/promotions/bone-fracture-risk/calculator/).31 These calculators use different clinical risk factors to calculate a risk score for individuals over the age of 50 years.32 Overall, both calculators have shown acceptable prediction of hip fractures but poorer prediction of major osteoporotic and fragility fractures.33
Additional tests
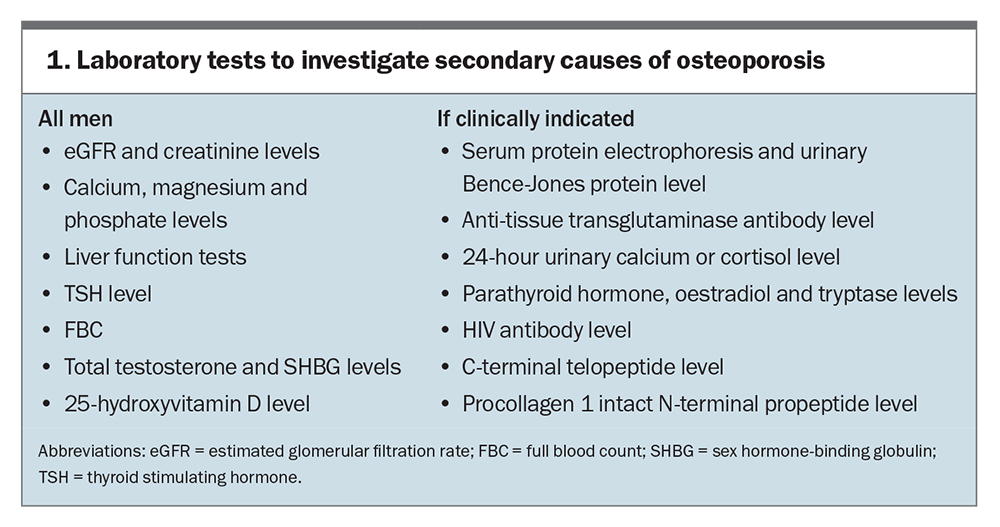
Routine tests include measurements of serum calcium, creatinine, 25-hydroxyvitamin D and thyrotropin levels, liver function tests and a complete blood count. When the Z-score is below −2.0 (two SDs below the age-specific mean), further laboratory testing to rule out secondary causes is strongly recommended (Box 1). If clinically indicated, additional tests, such as serum protein electrophoresis, urinary Bence-Jones protein, anti-tissue transglutaminase antibodies with IgA level, 24-hour urinary cortisol and calcium, serum parathyroid tryptase, oestradiol and HIV antibody levels, should be performed. Measurement of total testosterone is recommended for all men with osteoporosis, as hypogonadism is often difficult to detect based on history and physical examination alone. In men with no apparent cause of osteoporosis or with very low BMD, measuring bone turnover markers, such as C-terminal telopeptide of type 1 collagen and procollagen type 1 N, may be useful to detect low levels of bone formation.
Very high or imminent fracture risk
Men at very high or imminent fracture risk benefit from more rapid fracture risk reduction using anabolic therapy. These men should be identified for prompt specialist referral. Guidelines vary in the definition of very high fracture risk, but considerations include any or a combination of:
- two or more fragility fractures or vertebral fractures
- a T-score below −3 SD
- a recent fracture within two years
- a FRAX score of 30% or higher for a major osteoporotic fracture
- a FRAX score above 4.5% for a hip fracture.
Management
Both nonpharmacological and pharmacological therapies are important in the prevention and management of osteoporosis. Nonpharmacological approaches aim to reduce the risk of falls and help maintain BMD. Pharmacological therapies are recommended for men with a history of fractures or low BMD. Treatment decisions should be tailored to patients’ needs and risk profiles, and guided by evaluating absolute fracture risk, taking into account factors such as age, bone density and other relevant clinical considerations (Flowchart).34
Nonpharmacological therapy
Several general preventative and lifestyle factors are recommended for all men (Box 2). Maintaining balance and performing high-intensity progressive resistance exercises reduce the risk of falls and increase BMD among older adults.35 Observational data in older men also suggest a lower risk of fractures among those who maintain an active lifestyle.36 The benefits of high-intensity progressive resistance training appear to be primarily related to falls reduction rather than an increase in BMD.37
Calcium (≥1200 mg daily) and vitamin D (≥800 IU daily) supplementation have been found to reduce osteoporotic fractures by 12% in both men and women aged 50 years or older.38 The greatest reductions in fractures are observed in those with 80% or higher adherence rates and in older people who are institutionalised. Current recommendations support cholecalciferol supplementation of 800 to 2000 IU daily to maintain a serum 25-hydroxyvitamin D level of 75 ng/mL or higher, along with a daily intake of calcium of at least 1200 mg through diet or supplementation.39
Pharmacological therapy
Pharmacological therapy should be considered for all men with a history of hip or vertebral fractures, as well as those with a BMD T-score of −2.5 or lower at the spine or hip, based on their absolute fracture risk (Flowchart). Treatment should also be strongly considered for men with osteopenia and a nonvertebral minimal trauma fracture. According to recent guidelines from the National Osteoporosis Foundation, pharmacological therapy is recommended for men:
- aged 50 years or older with hip or vertebral fractures
- with a T-score −2.5 or lower
- with a T-score between −1.0 and −2.5 with a FRAX score of 3% or higher for hip fracture or 20% or higher for a major osteoporotic fracture.
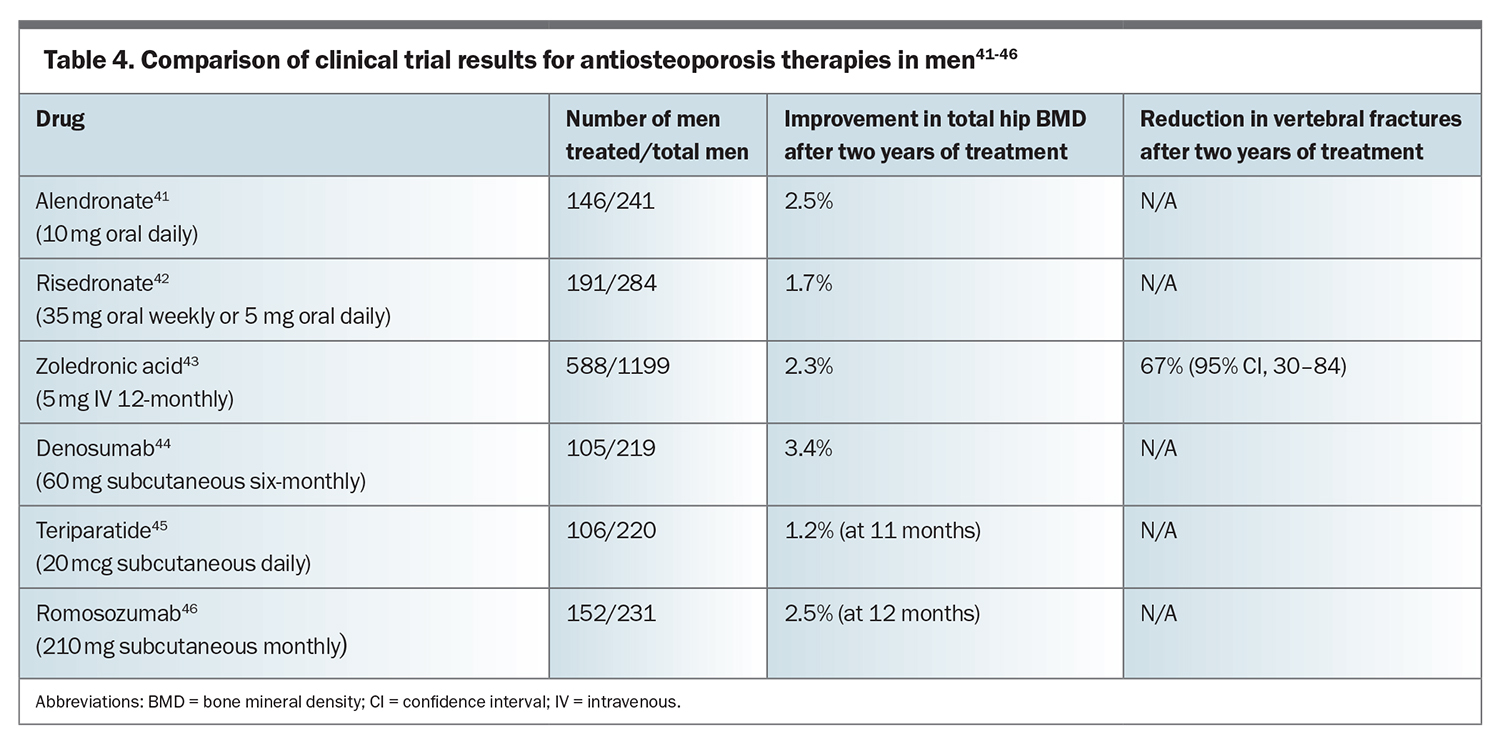
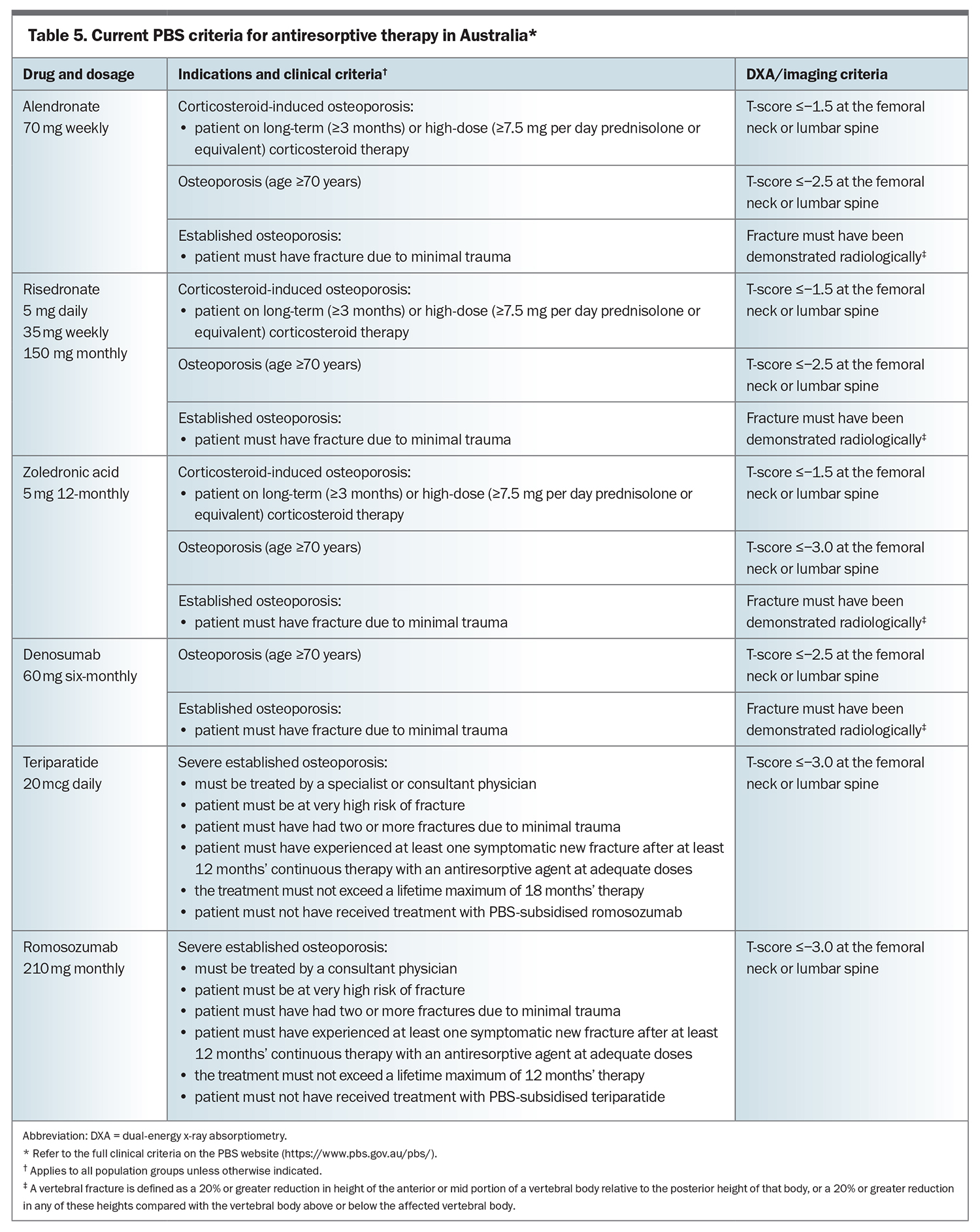
Furthermore, men on long-term systemic corticosteroid or antiandrogen therapy may benefit from antiosteoporosis therapy depending on their absolute fracture risk.40 The effects of antiosteoporosis drugs on BMD change and vertebral fracture risk are summarised in Table 4.41-46 The current PBS criteria for pharmacologic therapy for osteoporosis are summarised in Table 5.
Bisphosphonates
Bisphosphonates are approved as first-line antiresorptive drugs for the treatment of osteoporosis. Aminobisphosphonates inhibit the enzyme farnesyl pyrophosphate synthase in osteoclasts, resulting in inhibition of bone resorption. Bisphosphonates can be administered orally (weekly alendronate or daily, weekly or monthly risedronate) or intravenously (annual or 18-monthly zoledronic acid). A recent meta-analysis showed that bisphosphonates reduced the relative risk of vertebral and nonvertebral fractures by 63% and 40%, respectively, in men with osteoporosis.47
In one trial of 241 men with osteoporosis, who were either hypogonadal and eugonadal, treatment with alendronate 10 mg daily for two years increased BMD at the lumbar spine and femoral neck and significantly reduced the incidence of radiologic vertebral fractures compared with placebo (0.8% vs 7.1%).41 Another study that included 284 men with osteoporosis treated with risedronate 35 mg once weekly for two years showed a significant increase in BMD at the lumbar spine and total proximal femur (4.5% and 1.4%, respectively) at two years.42 However, fracture rates in both groups were similar, potentially due to the low incidence of fractures in the study. In a four-year extension of the original study, involving 218 of the initial participants, lumbar spine BMD continued to increase to 7.9% in those men who had taken risedronate for four years.48
The administration of zoledronic acid among older people, at one or two 5 mg doses within 90 days following hip fracture, resulted in a 35% reduction in overall rates of any new clinical fracture and a 28% reduction in death from any cause. However, no significant effects were seen on hip fracture rates over a 23-month study period.49 In another trial involving 1199 men with osteoporosis aged 50 to 85 years, two infusions of zoledronic acid 5 mg over 24 months significantly reduced the rate of new vertebral fractures by 67%. The treatment also led to fewer moderate to severe vertebral fractures, less height loss and an increase in BMD compared with the placebo group.43 A comparative study in men with osteoporosis receiving once-yearly zoledronic acid 5 mg versus once-weekly alendronate 70 mg demonstrated the noninferiority of zoledronic acid to alendronate. The study showed that 74.2% of men preferred the once-yearly zoledronic acid infusion over oral alendronate.50
Long-term bisphosphonate therapy has been associated with an increased risk of atypical femur fractures and osteonecrosis of the jaw, although the absolute risk for both remains extremely low. Therefore, in men who are not at high risk of minimal trauma fracture, it is reasonable to pause therapy for two years after five years of treatment with oral bisphosphonates or three years of treatment with intravenous zoledronic acid. However, in those with ongoing high risk of fractures, oral and intravenous bisphosphonate therapy may be continued for 10 years and six years, respectively.51 The beneficial effects of bisphosphonate therapy on BMD persist for several years after treatment with alendronate and zoledronic acid, but only lasts for about 12 months following risedronate therapy. It is important to measure BMD after two years of stopping treatment and consider restarting therapy if there has been a significant decline. Therapy may also need to be restarted earlier if a man’s risk profile has increased or if a fracture has been sustained.
Denosumab
Denosumab, a human antibody to receptor activator of the RANK ligand, is also considered a first-line treatment for osteoporosis. It works by inhibiting bone resorption through the decrease of osteoclast formation and differentiation, while also increasing osteoclast apoptosis. A study of 242 men with low BMD who received six-monthly subcutaneous injections of denosumab 60 mg for either 12 or 24 months showed significant improvements in BMD in the lumbar spine, total hip and distal radius.44 The BMD response to denosumab was similar to that seen in men with prostate cancer who experienced treatment-induced hypogonadism and had low bone mass or a history of fragility fractures. In these men, vertebral fractures were also reduced after 12 months of denosumab treatment.52
Recent studies have reported an increased incidence of multiple vertebral fractures when denosumab is discontinued or delayed for more than five weeks. This is likely due to a rapid increase in bone resorption and bone loss.53,54 Therefore, denosumab therapy should be continued indefinitely or, if discontinued, a replacement drug such as a bisphosphonate started six months after the last denosumab injection. Although the evidence is inconclusive, both alendronate and zoledronic acid appear to have greater efficacy for maintaining BMD after denosumab cessation compared with risedronate.55 However, BMD loss can still occur, with one study in postmenopausal women demonstrating an average BMD loss of 0.25 to 0.5 SD despite prompt treatment with zoledronic acid.56
Anabolic agents
Teriparatide
Teriparatide is a recombinant human parathyroid hormone (1-34) that exhibits an anabolic effect on bone by promoting net bone formation over bone resorption. In a study involving 437 men with low BMD, daily subcutaneous injections of teriparatide at doses of 20 or 40 mcg over a median period of 11 months resulted in an increase in spine BMD of 5.9% and 9.0%, respectively, and femoral neck BMD of 1.5% and 2.9%, respectively, compared with placebo.45 Teriparatide has also been shown to reduce the incidence of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis.57
A follow-up study of men initially treated with teriparatide and subsequently treated with antiresorptive therapy demonstrated a significant 83% reduction in the incidence of new moderate or severe vertebral fractures and a nonsignificant 51% risk reduction in vertebral fractures.58 Due to the development of bone osteosarcomas in rats, teriparatide therapy was initially limited to 24 months. However, as no significant increase in bone osteosarcomas was observed in postsurveillance studies, this limitation has been removed in the US, although it still remains in place in Australia.59
Under the PBS, the recommended duration of teriparatide treatment is 18 months for eligible patients with a T-score of −3.0 or less and at least two minimal trauma fractures, including a fracture occurring after at least one year of antiresorptive therapy. Following teriparatide treatment, antiresorptive therapy is initiated to maintain or further increase BMD. Despite being indicated as a second-line treatment under the PBS, teriparatide has shown to be more effective at increasing BMD when used as a first-line drug.
Romosozumab
Romosozumab is an antibody to sclerostin, which inhibits osteoblast function and bone formation. A trial involving 245 men aged 55 to 90 years with either a history of osteoporosis or osteopenia with a previous fragility fracture showed that subcutaneous romosozumab at a dose of 210 mg monthly for 12 months significantly increased BMD in the lumbar spine (12.1% vs 1.2%) and total hip (2.5% vs −0.5%) compared with placebo.46 Serum levels of procollagen type 1 N increased early in those receiving romosozumab, peaking at month 1; this corresponded with the greatest reduction in serum C-terminal telopeptide of type 1 collagen, which remained reduced throughout the remainder of the study. Notably, a small numerical difference in positively adjudicated cardiovascular serious adverse events was observed in the romosozumab-treated group compared with the placebo group (4.9% vs 2.5%). This effect was previously seen in a large trial comparing romosozumab with alendronate in postmenopausal women with osteoporosis (2.5% vs 1.9%), but not in another larger trial with a similar population that compared romosozumab with placebo.60,61
Romosozumab is contraindicated in men with a history of myocardial infarction or cerebrovascular accident in the past 12 months. Based on PBS criteria, it is only available for patients with at least two fragility fractures, a T-score of −3.0 or lower and at least one symptomatic new fracture after at least one year of antiresorptive therapy. Despite being indicated as a second-line treatment under the PBS, romosozumab has been shown to be more effective at increasing BMD when used as a first-line drug.
Superiority of anabolic drugs over antiresorptive drugs
Two recent studies in postmenopausal women with osteoporosis have demonstrated the superiority of anabolic drugs over oral bisphosphonates in reducing fractures. The VERO study showed that, over a two-year period, teriparatide was more effective than oral risedronate in reducing vertebral and clinical fractures.62 Similarly, the ARCH study demonstrated that 12 months of treatment with romosozumab followed by two years of oral alendronate was more effective than oral alendronate alone in reducing vertebral, nonvertebral, clinical and hip fractures.60 These studies provide compelling evidence for considering anabolic drugs in patients who are at a very high or imminent risk of fractures.
Testosterone
There are limited studies on testosterone therapy in men with osteoporosis and no studies have focused on fractures as a primary outcome. Hypogonadism affects up to 12.3% of men, and a study found that nearly 66% of older men aged 40 to 79 years in residential care with hip fractures had hypogonadism.63,64 In men aged over 65 years with hypogonadism, testosterone replacement therapy resulted in an 8.9% greater increase in spinal BMD compared with placebo over a period of three years.65 Another study showed that testosterone treatment for one year in men with hypogonadism aged 65 years and older significantly increased volumetric BMD, with greater gains observed in the spine compared with the hip and larger increases in trabecular bone compared with cortical bone.66 Previous research has also indicated improvements in trabecular connectivity in hypogonadal men undergoing testosterone therapy for two years.67 However, the effects of testosterone therapy in older eugonadal men are still controversial. One study found no benefit in BMD using transdermal testosterone compared with placebo over three years, while another study involving intramuscular testosterone injections over six months showed an improvement in spinal BMD and a decrease in bone remodelling.68-70 Due to the potential for serious side effects, testosterone therapy is not recommended for eugonadal men. However, for men without contraindications and at high risk of fractures, adjunctive testosterone replacement therapy should be considered alongside antiresorptive therapy to restore eugonadal status.
Conclusion
Despite advancements in antiosteoporosis therapy, osteoporosis in men remains underdiagnosed and undertreated. All men aged 70 years or older, or those who have experienced a minimal trauma fracture, should have a DXA scan to assess their BMD. Secondary causes should be excluded and treatment initiated if deemed appropriate. All men can benefit from general preventative and lifestyle measures, such as regular high-intensity progressive resistance training, ensuring adequate calcium intake, optimising vitamin D levels, avoiding smoking and excessive alcohol consumption and participating in fall prevention programs when applicable. Oral or intravenous bisphosphonates and subcutaneous denosumab are currently considered first-line treatments for osteoporosis in men. All men considering these therapies should be educated on their potential side effects. Furthermore, the use of anabolic therapy should be strongly considered in men with a very high or imminent risk of fractures. In such patients, prompt referral to a bone health specialist is recommended for further evaluation and appropriate management. ET
COMPETING INTERESTS: None.
References
1. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997; 7: 407-413.
2. Chang KP, Center JR, Nguyen TV, Eisman JA. Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo osteoporosis epidemiology study. J Bone Miner Res 2004; 19: 532-536.
3. Nguyen TV, Eisman JA, Kelly PJ, Sambrook PN. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol 1996; 144: 255-263.
4. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010; 152: 380-90.
5. Jiang HX, Majumdar SR, Dick DA, et al. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res 2005; 20: 494-500.
6. Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ. Epidemiology and clinical features of osteoporosis in young individuals. Bone 1994; 15: 551-555.
7. Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res 1992; 7: 221-227.
8. Jones G, White C, Nguyen T, Sambrook PN, Kelly PJ, Eisman JA. Prevalent vertebral deformities: relationship to bone mineral density and spinal osteophytosis in elderly men and women. Osteoporos Int 1996; 6: 233-239.
9. O’Neill TW, Felsenberg D, Varlow J, et al. The prevalence of vertebral deformity in European men and women: The european vertebral osteoporosis study. J Bone Miner Res 1996; 11: 1010-1018.
10. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 2011; 26: 1729-1739.
11. Walsh JS, Henry YM, Fatayerji D, Eastell R. Lumbar spine peak bone mass and bone turnover in men and women: a longitudinal study. Osteoporos Int 2009; 20: 355-362.
12. Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass. Osteoporos Int 1994; 4 Suppl 1: 7-13.
13. Nieves JW, Formica C, Ruffing J, et al. Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res 2005; 20: 529-535.
14. Nordström P, Neovius M, Nordström A. Early and rapid bone mineral density loss of the proximal femur in men. J Clin Endocrinol Metab 2007; 92: 1902-1908.
15. Szulc P, Delmas PD. Biochemical markers of bone turnover in men. Calcif Tissue Int 2001; 69: 229-234.
16. Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006; 91: 3908-3915.
17. Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 2008; 23: 205-214.
18. Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 2006; 21: 124-131.
19. Silva MJ, Gibson LJ. Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone 1997; 21: 191-199.
20. Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol 2002; 167: 1952-1956.
21. Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adults. J Bone Miner Res 2008; 23: 143-150.
22. Lewiecki EM, Lane NE. Common mistakes in the clinical use of bone mineral density testing. Nat Clin Pract Rheumatol 2008; 4: 667-674.
23. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312: 1254-1259.
24. The relationship between bone density and incident vertebral fracture in men and women. J Bone Miner Res 2002; 17: 2214-2221.
25. de Laet CE, van der Klift M, Hofman A, Pols HA. Osteoporosis in men and women: a story about bone mineral density thresholds and hip fracture risk. J Bone Miner Res 2002; 17: 2231-2236.
26. Johnell O, Kanis J, Gullberg G. Mortality, morbidity, and assessment of fracture risk in male osteoporosis. Calcif Tissue Int 2001; 69: 182-184.
27. Pasco JA, Lane SE, Brennan SL, et al. Fracture risk among older men: osteopenia and osteoporosis defined using cut-points derived from female versus male reference data. Osteoporos Int 2014; 25: 857-862.
28. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005; 16: 581-589.
29. Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo osteoporosis epidemiology study. Arch Intern Med 2008; 168: 47-54.
30. Sambrook PN, Seeman E, Phillips SR, Ebeling PR. Preventing osteoporosis: outcomes of the Australian Fracture Prevention Summit. Med J Aust 2002; 176: S1-16.
31. Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int 2008; 19: 1431-1444.
32. Stuckey B, Magraith K, Opie N, Zhu K. Fracture risk prediction and the decision to treat low bone density. Aust J Gen Pract 2021; 50: 165-170.
33. Holloway-Kew KL, Zhang Y, Betson AG, et al. How well do the FRAX (Australia) and Garvan calculators predict incident fractures? Data from the Geelong Osteoporosis Study. Osteoporos Int 2019; 30: 2129-2139.
34. Kanis JA, Black D, Cooper C, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int 2002; 13: 527-536.
35. Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. BMJ 2004; 328: 680.
36. Michaëlsson K, Olofsson H, Jensevik K, et al. Leisure physical activity and the risk of fracture in men. PLoS Med 2007; 4: e199.
37. Allison SJ, Folland JP, Rennie WJ, Summers GD, Brooke-Wavell K. High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone 2013; 53: 321-328.
38. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007; 370: 657-666.
39. Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 2001; 73: 288-294.
40. The Royal Australian College of General Practitioners and Osteoporosis Australia. Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age. 2nd edn. East Melbourne, Vic: RACGP, 2017.
41. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000; 343: 604-610.
42. Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 2009; 24: 719-725.
43. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 2012; 367: 1714-1723.
44. Langdahl BL, Teglbjærg CS, Ho PR, et al. A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 2015; 100: 1335-1342.
45. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003; 18: 9-17.
46. Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 2018; 103: 3183-3193.
47. Nayak S, Greenspan SL. Osteoporosis treatment efficacy for men: A systematic review and meta-analysis. J Am Geriatr Soc 2017; 65: 490-495.
48. Boonen S, Lorenc RS, Wenderoth D, Stoner KJ, Eusebio R, Orwoll ES. Evidence for safety and efficacy of risedronate in men with osteoporosis over 4 years of treatment: results from the 2-year, open-label, extension study of a 2-year, randomized, double-blind, placebo-controlled study. Bone 2012; 51: 383-388.
49. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357: 1799-1809.
50. Orwoll ES, Miller PD, Adachi JD, et al. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 2010; 25: 2239-2250.
51. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American society for bone and mineral research. J Bone Miner Res 2016; 31: 16-35.
52. Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361: 745-755.
53. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011; 96: 972-980.
54. Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: A post hoc analysis of the randomized placebo-controlled FREEDOM trial and Its extension. J Bone Miner Res 2018; 33: 190-198.
55. Tay WL, Tay D. Discontinuing denosumab: can it be done safely? A review of the literature. Endocrinol Metab (Seoul) 2022; 37(2): 183-194.
56. Sølling AS, Harsløf T, Langdahl B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res 2020; 35: 1858-1870.
57. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434-1441.
58. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 2005; 16: 510-516.
59. Krege JH, Gilsenan AW, Komacko JL, Kellier-Steele N. Teriparatide and osteosarcoma risk: History, science, elimination of boxed warning, and other label updates. JBMR Plus 2022; 6: e10665.
60. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017; 377: 1417-1427.
61. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532-1543.
62. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018; 391: 230-240.
63. Mohr BA, Guay AT, O’Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts male ageing study. Clin Endocrinol (Oxf) 2005; 62: 64-73.
64. Abbasi AA, Rudman D, Wilson CR, et al. Observations on nursing home residents with a history of hip fracture. Am J Med Sci 1995; 310: 229-234.
65. Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 2004; 89: 503-510.
66. Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: A controlled clinical trial. JAMA Intern Med 2017; 177: 471-479.
67. Benito M, Vasilic B, Wehrli FW, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res 2005; 20: 1785-1791.
68. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999; 84: 1966-1972.
69. Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis-effects of 6 months of treatment on bone mineral density and cardiovascular risk factors. Bone 1996; 18: 171-177.
70. Anderson FH, Francis RM, Peaston RT, Wastell HJ. Androgen supplementation in eugonadal men with osteoporosis: effects of six months’ treatment on markers of bone formation and resorption. J Bone Miner Res 1997; 12: 472-478.