Individualised management of osteoporosis: choosing the right drug for the right patient

Osteoporosis is a chronic skeletal disorder associated with increased risk of minimal trauma fractures and poses a significant health burden. The initiation of osteoporosis pharmacotherapy for fracture risk reduction is most often performed in primary care and various treatment options are available, each with their own bone-strengthening efficacy, side-effect profiles and duration of action. This article describes three common clinical scenarios of patients with osteoporosis requiring treatment initiation, discusses the rationale between different treatment choices and provides practical advice on counselling of patients.
- Osteoporosis is characterised by loss of bone mass, leading to increased susceptibility of minimal trauma or fragility fractures.
- Osteoporosis treatments are broadly categorised as antiresorptive (bisphosphonates, denosumab) or osteoanabolic (romosozumab, teriparatide).
- When individualising osteoporosis therapies, several considerations arise, including patient age, treatment targets, baseline fracture risk, presence of a secondary cause, risk of side effects, patient preference and adherence.
- Bisphosphonates and denosumab are effective first-line therapies for improving bone mineral density and reducing risk of fracture.
- Osteoanabolic agents can be considered in people with more severe osteoporosis and this requires specialist input and monitoring. Osteoanabolic agents should be followed by a suitable antiresorptive agent.
- Treatment breaks can be considered in patients with no interval fractures and improved bone mineral density with low ongoing risk of fracture after a course of bisphosphonate treatment.
- Treatment breaks should be avoided with denosumab. Denosumab cessation is associated with rebound bone loss and hence delayed or missed doses should be avoided and specialist input is recommended if cessation is being considered.
Osteoporosis is characterised by low bone mass and microarchitectural deterioration, with increased risk of minimal trauma or fragility fractures (from impact equal to or less than a fall from standing height). Osteoporosis is common, with about one in three women and one in five men older than 50 years of age experiencing an osteoporotic fracture in their remaining lifetime.1 After a first fracture, the risk of subsequent fractures increases significantly, prompting an urgent need to recognise and treat such patients.2-4
Treatment options for osteoporosis can be broadly categorised into antiresorptive (e.g. bisphosphonates, denosumab) and osteoanabolic medications (teriparatide, romosozumab). Although guidelines assist clinicians with deciding which patients are most suitable to screen, diagnose and treat for osteoporosis, there is less direction regarding appropriate choice of pharmacotherapy. This article discusses three common clinical scenarios of older adults requiring osteoporosis pharmacotherapy for fracture risk reduction and the rationale between different treatment choices and also provides practical advice on counselling of patients regarding different options and associated risks.
Case 1. Bisphosphonates
A 56-year-old woman presents in general practice with a recent left distal radius fracture following a fall from standing height. Her past medical history is unremarkable. She underwent menopause in her early 50s, has no underlying chronic inflammatory conditions, and does not take any medications including corticosteroids. She is a nonsmoker and has minimal alcohol consumption. She is encouraged to increase calcium intake to three servings daily and cholecalciferol supplementation is started because her vitamin D level is below 50 nmol/L. A recent dual energy x-ray absorptiometry (DXA) scan indicates lumbar spine bone mineral density (BMD) T-score of –2.4 SD, left total hip T-score of –2.1 SD and left femoral neck T-score of –2.3 SD.
Discussion
This is a common scenario in which a peri/postmenopausal woman presents with her first minimal trauma fracture. Patients who present with a minimal trauma fracture are at increased risk of subsequent fractures, particularly over the next few years and, thus, timely assessment and secondary fracture prevention are crucial.2,4,5
Optimisation of dietary and lifestyle factors is important in osteoporosis management. The optimal dietary calcium intake is 1300 mg/day for women older than 50 years and men older than 70 years of age, and 1000 mg for men aged 50 to 70 years.6 Three daily servings of dairy or other calcium-rich foods are recommended, and calcium supplementation may be required if dietary intake is insufficient. Adequate and safe sunlight exposure is recommended, and cholecalciferol supplementation may be needed to maintain vitamin D levels of 50 nmol/L or more.6,7 Physical activity such as progressive resistance training and high impact weight-bearing exercises are recommended for improving bone strength.8,9
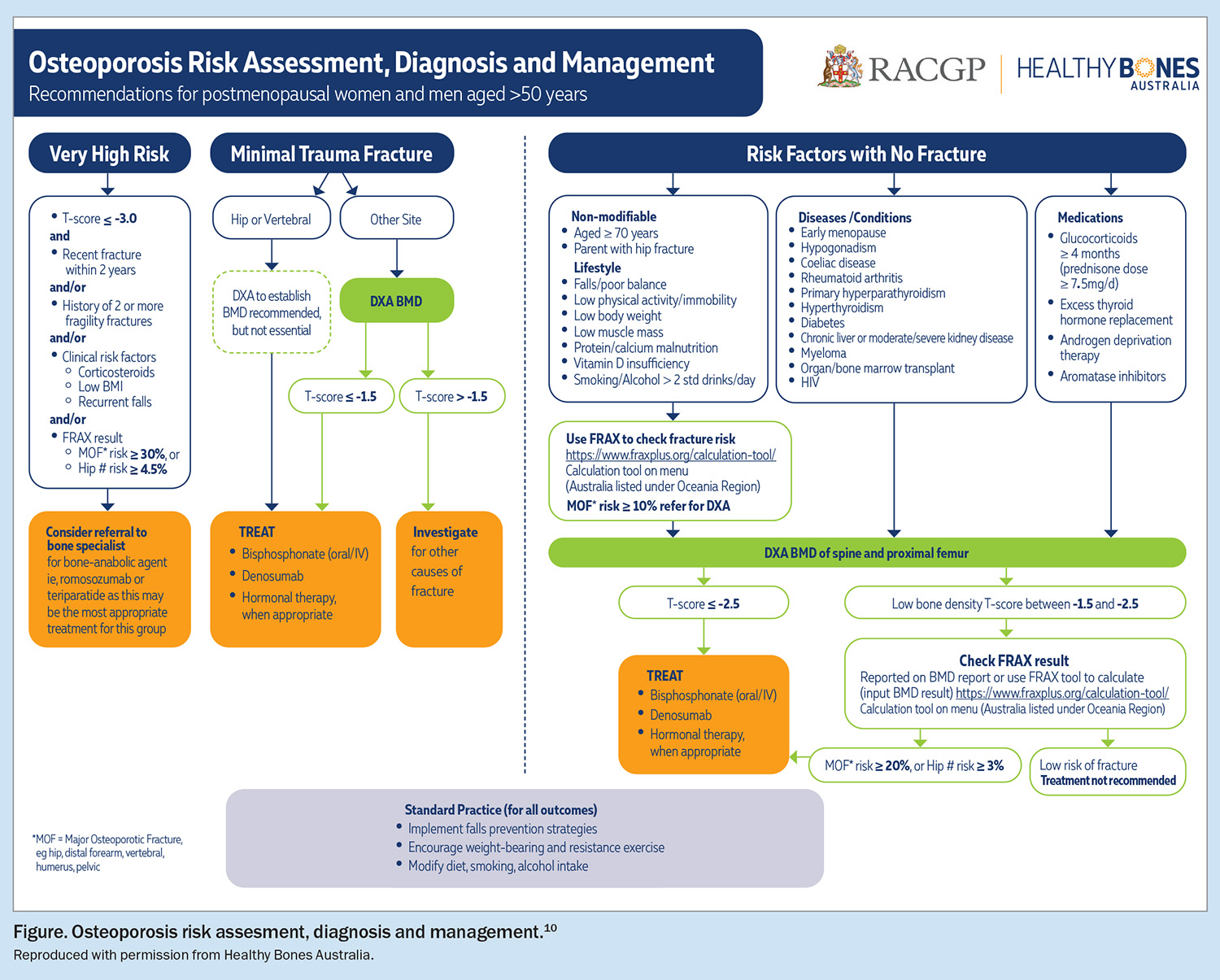
This patient is suitable for osteoporosis treatment based on recently updated 2024 Royal Australian College of General Practitioners guidelines on osteoporosis management in postmenopausal women and men aged over 50 years (Figure).10 She has a recent history of a fragility fracture and exhibits BMD in the osteopenic range, which is expected to decline by a further 10% during the 10 years postmenopause.11 Various treatment approaches, including menopausal hormone therapy (MHT), bisphosphonates, denosumab and osteoanabolic therapy, were discussed with the patient. MHT is associated with a 33% reduction in vertebral fractures and 27% reduction in nonvertebral fractures.12,13 MHT is suitable as initial osteoporosis therapy in women less than 10 years postmenopause who also require treatment for vasomotor symptoms and women who have undergone early menopause.14 This patient lacked vasomotor symptoms and preferred to commence oral bisphosphonates. Either oral or intravenous bisphosphonates are appropriate given her relatively young age (Box 1). It is important to note that the patient has osteoporosis based on the presence of a minimal trauma fracture, despite her BMD being in the osteopenic range. In general, patients may be treated with at least three years of intravenous bisphosphonates and at least five years of oral bisphosphonates before considering a treatment break.15 However, prolonged therapy is required for patients who remain at high risk of ongoing fracture, and further in-depth discussion on considerations of treatment breaks can be found elsewhere.15
Efficacy and safety of bisphosphonates
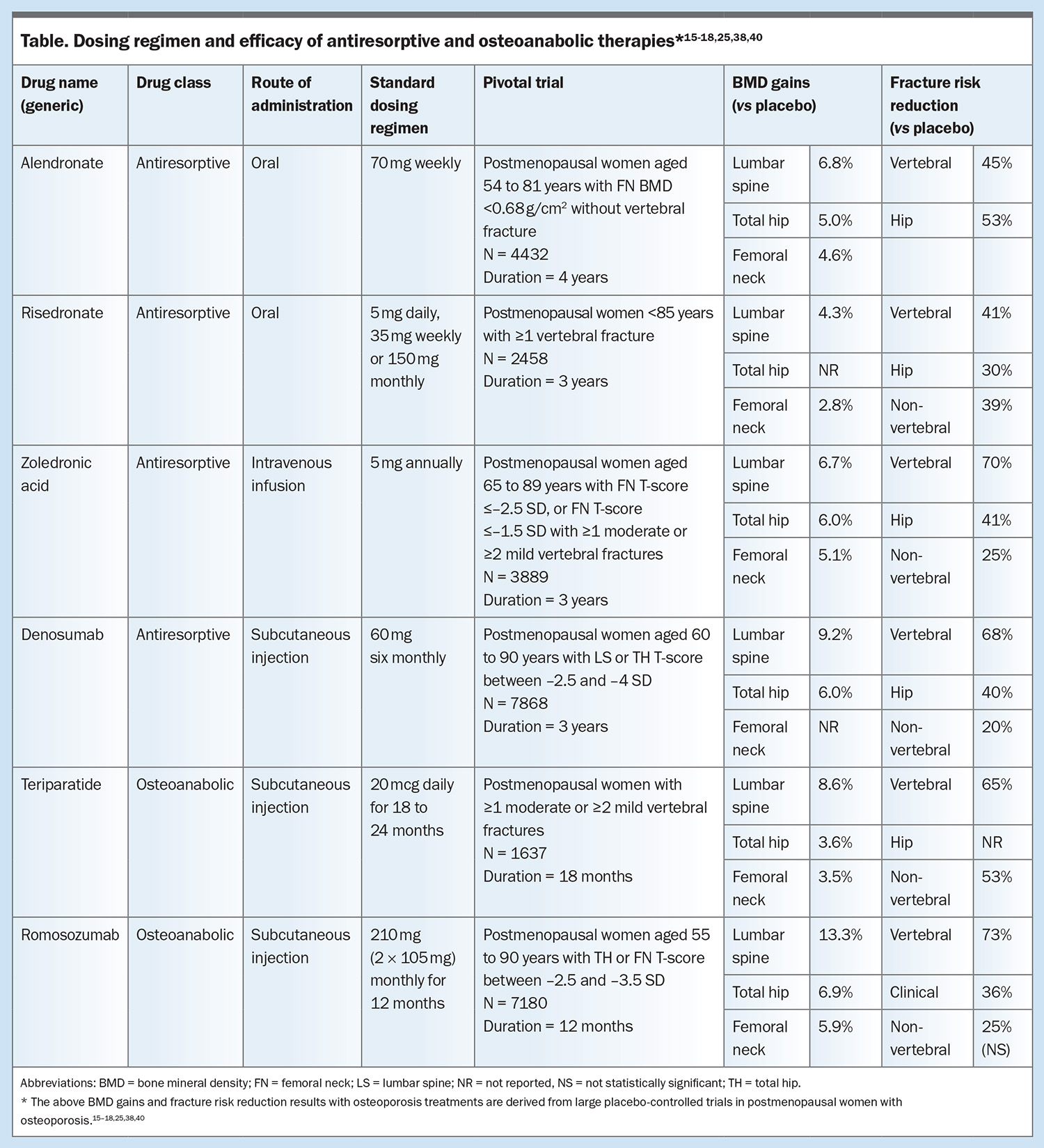
In women with low BMD but without vertebral fractures, use of alendronate for four years led to BMD gains of 4.6% at the femoral neck, 5.0% at the total hip and 6.8% at the lumbar spine compared with placebo.16 In the Fracture Intervention Trial, the risk of hip fracture was reduced by 53% and clinical vertebral fracture by 45% in women with osteoporosis who took alendronate for three to four years.17 In comparison, use of risedronate for three years increased BMD by 2.8% at the femoral neck and 4.3% at the lumbar spine and reduced vertebral fractures by 41% and nonvertebral fractures by 39% in postmenopausal women with established osteoporosis.18 After three years of annual zoledronic acid infusions, BMD was shown to increase by 6.0% at the total hip, 5.1% at the femoral neck and 6.7% at the lumbar spine in postmenopausal women with osteoporosis.19 Risk of vertebral, hip and nonvertebral fractures decreased by 70%, 41%, and 25%, respectively (Table.)
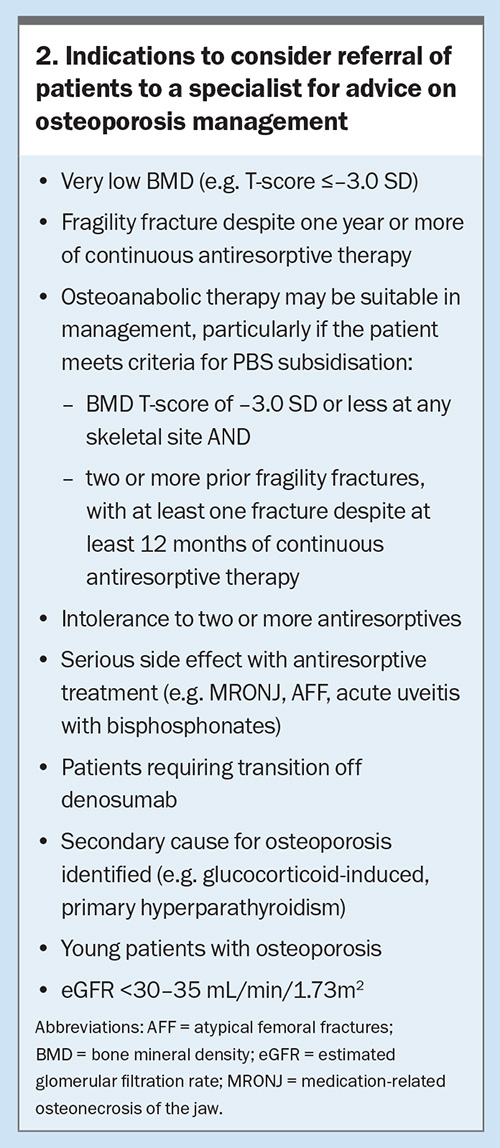
Oral bisphosphonates should be avoided in patients with dysphagia, oesophagitis, symptomatic reflux and peptic ulcer disease. Patients should also be counselled on correct administration, including remaining upright and avoiding food or other medications affecting absorption for 30 minutes after ingestion. Enteric-coated risedronate may be taken with food. Zoledronic acid is usually administered as a once yearly infusion, although it has also been shown to reduce the risk of fracture if administered 18 monthly in lower risk patients.20 The intravenous route mitigates the risk of gastrointestinal side effects, poor absorption and adherence issues. About one-third of patients may experience an acute phase reaction with the first infusion, which is much less likely on subsequent infusions.19 Patients may experience onset of fever, fatigue and musculoskeletal discomfort the day after the infusion, which is usually mild and transient (lasting less than three days). All patients should be counselled regarding risk of an infusion reaction. We recommend liberal oral hydration and up to 1 g paracetamol four times daily for three days, starting on the day of the infusion. As bisphosphonates are renally excreted, they are not recommended in patients with creatinine clearance of less than 30 to 35 mL/min, particularly zoledronic acid;21 these patients should be referred to a specialist for treatment decisions (Box 2).
Atypical femoral fractures (AFFs) occurring in the mid-shaft or subtrochanteric shaft of the femur are a rare complication of long-term bisphosphonate use.22 Ten-year follow-up data showed that for every one AFF that occurred due to bisphosphonate use in Caucasian women, over 35 hip fractures were prevented.23 The benefit/risk ratio is therefore in favour of using bisphosphonates to prevent fragility hip fractures, but this ratio becomes smaller with prolonged therapy. The incidence of medication-related osteonecrosis of the jaw (MRONJ) in the setting of osteoporosis treatment is very low (less than one in 10,000 patient-years).24 In low-risk patients with a hip T-score greater than –2.5 SD and no new fractures during treatment, a treatment break with ongoing BMD monitoring may be appropriate.15 The risk of AFF decreases by 70% per year off treatment and thus returns to baseline within a few years.25 It is important to note that these patients will likely need to restart therapy in the future, for example, if fracture risk increases, BMD declines significantly or they sustain a new fracture.
Case 2. Denosumab
An 80-year-old woman presents to her GP for management of her osteoporosis. Five years ago, she sustained a right proximal humerus fracture after a fall, which was surgically managed but she did not receive any osteoporosis treatment at that stage. Three months ago, she had a fall while walking outdoors and sustained a left neck of femur fracture, which required hospitalisation for a left hip hemiarthroplasty. A subsequent DXA scan revealed T-scores of –2.8 SD at the lumbar spine, –2.2 SD at the right total hip and –2.4 SD at the right femoral neck. She lives with her husband at home, remains independent with daily activities, is a nonsmoker and has minimal alcohol intake. She has regular six-monthly dental review with no recent or pending dental procedures. Her vitamin D is replete (85 nmol/L) and renal function is safe for commencing treatment (estimated glomerular filtration rate of 60 mL/min/1.73 m2).
Discussion
This patient has a high future risk of fracture, given two prior fragility fractures (including a recent hip fracture) and low BMD T-scores. She is suitable for osteoporosis treatment to reduce her risk of fracture. Various treatment options, including oral and intravenous bisphosphonates, subcutaneous denosumab and osteoanabolic therapy, are discussed with the patient. Denosumab is considered the most suitable option because of her older age, recent hip fracture and high ongoing fracture risk, which make the need for long-term continuous treatment likely (Box 3).
Before the patient starts denosumab, she is informed of the potential side effects, including hypocalcaemia and rare skeletal complications, such as AFF and MRONJ, with MRONJ risk considered particularly low because she maintains good dental hygiene. Dental review may be appropriate before starting treatment if the patient is at high risk of MRONJ and this does not significantly delay starting antiosteoporosis therapy. She is also counselled on the risk of rebound bone loss after denosumab cessation.
The patient starts six-monthly injections and ongoing injections are scheduled with her local medical practice to ensure doses are not delayed or missed. Standard dietary and lifestyle advice are also provided (as above for bisphosphonate therapy).
The patient experienced a robust BMD response to denosumab over subsequent years with no further fragility fracture, and BMD monitoring was continued every two to three years.
Efficacy and safety of denosumab
Denosumab is a potent antiresorptive administered as six-monthly 60 mg subcutaneous injections by a healthcare professional. Recently, denosumab has become the most common newly prescribed osteoporosis treatment in Australia, largely because of its convenient mode of administration and favourable long-term efficacy and safety.26 In postmenopausal women with osteoporosis, denosumab given subcutaneously six monthly for three years was associated with an increase of 9.2% in BMD at the lumbar spine and 6.0% at the total hip, compared with placebo.27 It also reduced the risk of vertebral fractures by 68%, hip fractures by 40% and nonvertebral fractures by 20%.27 Longer-term denosumab use for up to 10 years showed sustained reductions in fracture risk and ongoing progressive improvements in BMD by 21.7% at the lumbar spine and 9.2% at the total hip.28
Long-term safety of denosumab is reassuring, with very low rates of AFF and MRONJ. It is estimated that 281 and 40 clinical fractures are prevented for every case of AFF and MRONJ, respectively, which can be a useful way to describe this risk to patients.29 Hence, although such skeletal complications can be serious, they are rare, and patients should be informed but also largely reassured of these risks. Detailed recommendations on preventive measures and patient counselling regarding AFF and MRONJ can be found elsewhere.30 Transient hypocalcaemia occurred in up to 8% of postmenopausal women with osteoporosis treated with denosumab in a real-world study, despite most patients having sufficient vitamin D status.31 Risk factors for hypocalcaemia include lower pretreatment calcium levels, vitamin D deficiency and worse renal function.31 All patients should have calcium and vitamin D levels checked and deficiencies corrected before commencing denosumab. Vitamin D levels should be 50 nmol/L or more before initiation and maintained above this level during treatment. Monitoring of vitamin D levels thereafter can be undertaken at least yearly, or more frequently if vitamin D deficiency is suspected. If there are concerns that the patient is at particularly high risk of hypocalcaemia then serum calcium levels can be measured two to three weeks after the denosumab dose.
Rebound bone loss after denosumab cessation
Unlike for bisphosphonates, treatment breaks should be avoided for patients receiving denosumab for osteoporosis. Although data on continuous BMD gains, persistent fracture risk reduction and long-term safety have only been published with up to 10 years of denosumab exposure, continuing treatment beyond this timeframe remains a suitable approach, particularly in patients with ongoing high fracture risk. An even more compelling reason to avoid treatment breaks with denosumab is that cessation triggers an early rebound increase in bone turnover, resulting in rapid loss of BMD gains by 18 months after the last dose.32
Denosumab may be ceased, however, under specific circumstances, which include patient preference or intolerance, low ongoing fracture risk without the need for long-term therapy or the occurrence of AFF or MRONJ. To minimise BMD loss following denosumab withdrawal, a bisphosphonate should be commenced. The optimal choice, timing and duration of bisphosphonate transition is an area of ongoing investigation and hence these patients should be referred to osteoporosis specialists. This approach may not be suitable, however, if denosumab is being ceased because of the occurrence of a serious skeletal adverse event, such as AFF or MRONJ. In such complex cases, semi-urgent specialist review should be sought to consider the risk of fragility fracture with cessation of denosumab versus the risk of progression or recurrence of AFF or MRONJ with continuing denosumab use.33,34 In cases where denosumab cessation is advised by a dental professional before an elective dental procedure, it may be prudent to perform the procedure four to five months after the last denosumab dose and restart treatment as soon as the lesion has healed, to minimise treatment interruption.33 It is important that denosumab treatment is not delayed for more than four weeks from the missed dose. Before commencing denosumab, all patients should be adequately informed of the risk of rebound bone loss and the requirement for long-term continuous treatment, given that treatment breaks are not advised.
The most suitable patients for commencing denosumab are those who are older with high risk of fracture, such that continuing treatment indefinitely would have a high benefit/risk ratio, and those who demonstrate good adherence and engagement with their healthcare team, such that risk of treatment interruption or cessation is reduced. Ongoing denosumab adherence tends to decline during the second year of treatment and additional patient education at this time is important.35 The use of electronic patient reminder systems, which are effective for improving adherence, are recommended to avoid unplanned interruptions to treatment.36
Case 3. Osteoanabolic therapy
A 65-year-old woman presents for follow up in general practice after hospital discharge for management of her osteoporosis. Five years ago, she sustained a right distal radius fracture after a fall. A DXA BMD scan showed a lowest T-score of –3.4 SD at the lumbar spine and she was commenced on risedronate 35 mg weekly. Progress DXA scans demonstrated minimal improvement in BMD. She recently presented to the emergency department with acute lower back pain after slipping and falling in her bathroom and was diagnosed with an L2 compression fracture. A subsequent DXA scan showed T-scores of –3.1 SD at the lumbar spine and –2.7 SD at the total hip. She confirmed good adherence with oral bisphosphonate therapy. Further assessment did not identify any secondary causes for osteoporosis. There is no history of cardiovascular disease.
Discussion
This patient has a very high risk of future fracture because of her very low T-scores and a prevalent fracture history (including a symptomatic vertebral fracture) despite five years of oral bisphosphonate use. Various treatment options, including intravenous bisphosphonates, subcutaneous denosumab and osteoanabolic therapy, are discussed with the patient. Although each option could be appropriate, osteoanabolic therapies are recommended in patients with a very high risk of fracture and have been shown to be superior to antiresorptives regarding both BMD gains and fracture risk reduction.37,38 Furthermore, this patient qualifies for PBS-funded osteoanabolic therapy, therefore, cost of treatment will be significantly reduced (Box 4).
The patient was subsequently referred to an osteoporosis specialist who initiated romosozumab. She had a significant improvement in BMD following 12 months of romosozumab use after which she was switched to ongoing zoledronic acid infusions to consolidate her BMD gains.
Role of osteoanabolic therapy and available options
Osteoanabolic therapies available in Australia are romosozumab and teriparatide. Both agents act by predominantly stimulating bone formation (as opposed to antiresorptives that curb bone resorption) and are associated with rapid gains in BMD and robust reductions in risk of fracture. Romosozumab also inhibits bone resorption.
All patients receiving osteoanabolics should be managed by an osteoporosis specialist. However, GPs play an important role in referring suitable patients for potential commencement of osteoanabolic therapy and should be familiar with safety concerns during treatment. These treatments are recommended for patients who have a very high risk of fracture before commencing antiresorptives (first line) or can be used as treatment escalation after initial antiresorptive treatment (second line), as described in this case. Romosozumab and teriparatide are TGA approved in either setting; however, at this stage they are only subsidised on the PBS as second-line treatment for patients who are at very high risk of fracture, have a BMD T-score of –3.0 SD or less and have had two or more fractures due to minimal trauma, including at least one symptomatic new fracture after at least 12 months of continuous therapy with an antiresorptive agent. Following an osteoanabolic treatment course, subsequent treatment with an antiresorptive is recommended to maintain skeletal benefits.39,40
Efficacy and safety of romosozumab
Romosozumab is administered as 210 mg monthly subcutaneous injections for 12 months by a healthcare professional, using two single-use prefilled syringes of 105 mg. Initial prescription for the first six months is by a specialist, while ongoing prescription for the last six months can be made by a GP. Romosozumab has demonstrated greater BMD gains and fracture risk reduction compared with placebo and oral alendronate. Use of romosozumab in postmenopausal women with osteoporosis led to BMD gains of 13.3% at the lumbar spine, 6.9% at the total hip and 5.9% at the femoral neck, compared with placebo, and resulted in a 73% lower risk of vertebral fractures and 36% lower risk of clinical fractures.40 Over a period of 24 months, treatment with romosozumab followed by alendronate resulted in a 48% lower risk of new vertebral fractures than alendronate alone.37 BMD gains appear to be most pronounced in patients receiving romosozumab as first-line treatment, whereas such dramatic gains are less common in patients with prior antiresorptive treatment.41
Romosozumab is generally well tolerated with serious side effects being rare. The most common side effects include transient nasopharyngitis and musculoskeletal discomfort (about 10% of patients) and mild injection site reactions (about 5% of patients).37,41 There was no difference in cardiovascular risk with romosozumab compared with placebo; however, serious cardiovascular adverse events occurred slightly more frequently compared with alendronate (2.5% vs 1.9%).37,41 It is unclear whether romosozumab use is associated with increased risk of cardiovascular disease or whether these findings were due to chance. To exercise caution, the TGA recommends that romosozumab be avoided in patients with a history of myocardial infarction or stroke. Given this patient had no history of cardiovascular disease, this was not a contraindication to its use. We also consider it good practice to assess for and address any modifiable cardiovascular risk factors when commencing romosozumab.
Efficacy and safety of teriparatide
Teriparatide can be self-administered as a 20 mcg daily subcutaneous injection using a pen containing a prefilled cartridge. The maximal treatment duration is 24 months, although PBS reimbursement can only be obtained for up to 18 months (i.e. the last six months of treatment, if required, would need to be self-funded). Teriparatide has led to a greater reduction in risk of fracture compared with placebo (65% lower risk of vertebral fractures and 53% lower risk of nonvertebral fractures) and risedronate (56% lower risk of vertebral fractures).38,42
Teriparatide is usually well tolerated. The most common side effects include mild transient hypercalcaemia (about 10% of patients), headache, nausea, dizziness or musculoskeletal discomfort (<5 to 10% of patients). Concerns regarding osteosarcoma were initially raised based on preclinical studies in rats; however, 15-year postmarketing surveillance data from the US reassuringly did not demonstrate an increased risk in humans.43,44 Nonetheless, in Australia, teriparatide is contraindicated in patients with risk factors for osteosarcoma, including unexplained elevated alkaline phosphatase, Paget’s disease or prior skeletal irradiation. Teriparatide is also a useful alternative to romosozumab in patients who are suitable for osteoanabolic therapy but have a cardiovascular contraindication to romosozumab.
Conclusion
Osteoporosis is a chronic skeletal condition associated with increased risk of fracture and is a rising burden on our healthcare system and in our ageing communities. GPs play crucial roles in identifying suitable patients and initiating them on appropriate pharmacotherapy and recognising who may benefit from specialist referral. Clinicians can be guided in their treatment decisions by a greater understanding of the various treatment options available, their efficacy regarding BMD gains and fracture risk reduction, suitability of treatment breaks, and specific safety considerations with both long-term treatment and treatment cessation. ET
COMPETING INTERESTS: Dr Wang: None. Dr Kumar has received payment from Kyowa Kirin for being on the Advisory Board. Associate Professor Wong is Honorary Medical Director and Board Member at Healthy Bones Australia. Associate Professor Girgis has received an honorarium from Gedeon Richter.
References
1. Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol 2017; 4: 46-56.
2. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007; 297: 387-394.
3. Center JR. Fracture Burden: What Two and a Half Decades of Dubbo Osteoporosis Epidemiology Study Data Reveal About Clinical Outcomes of Osteoporosis. Curr Osteoporos Rep 2017; 15: 88-95.
4. Johnell O, Kanis JA, Oden A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int 2004; 15: 175-179.
5. Giangregorio LM, Leslie WD, Manitoba Bone Density P. Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res 2010; 25: 1400-1405.
6. National Health and Medical Research Council AGDoHaA, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand. Canberra: National Health and Medical Research Council; 2006.
7. Nowson CA, Mason RS. Vitamin D and health in adults in Australia and New Zealand. Med J Aust 2013; 199: 394.
8. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 2011(7): CD000333.
9. Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 1): A systematic review. Bone 2021; 143: 115696.
10. Royal Australian College of General Practitioners, Healthy Bones Australia. Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age. Royal Australian College of General Practitioners; March 2024.Available online at: https://healthybonesaustralia.org.au/wp-content/uploads/2024/03/hba-racgp-guidelines-2024.pdf (accessed April 2024).
11. Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 2012; 27: 111-118.
12. Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA 2001; 285: 2891-2897.
13. Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trials. BMC Musculoskelet Disord 2001; 2: 7.
14. UK, NCCfWsaCsH. Menopause: Full Guideline Menopause: Full Guideline. National Institute for Health and Care Excellence: Clinical Guidelines. London2015.
15. Wang M, Wu YF, Girgis CM. Bisphosphonate Drug Holidays: Evidence From Clinical Trials and Real-World Studies. JBMR Plus 2022; 6(6): e10629.
16. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280: 2077-2082.
17. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000; 85: 4118-4124.
18. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282: 1344-1352.
19. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809-1822.
20. Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 2018; 379: 2407-2416.
21. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008; 83: 1032-1045.
22. Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use. N Engl J Med 2010; 362: 1848-1849.
23. Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med 2020; 383: 743-753.
24. Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22: 1479-1491.
25. Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364: 1728-1737.
26. Naik-Panvelkar P, Norman S, Elgebaly Z, et al. Osteoporosis management in Australian general practice: an analysis of current osteoporosis treatment patterns and gaps in practice. BMC Fam Pract 2020; 21(1): 32.
27. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756-765.
28. Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5: 513-523.
29. Ferrari S, Lewiecki EM, Butler PW, et al. Favorable skeletal benefit/risk of long-term denosumab therapy: a virtual-twin analysis of fractures prevented relative to skeletal safety events observed. Bone 2020; 134: 115287.
30. Kumar S, Diamond T. Osteoporosis: a practical update on its management Endocrinology Today 2022; 23(10): 36-48.
31. Tsvetov G, Amitai O, Shochat T, Shimon I, Akirov A, Diker-Cohen T. Denosumab-induced hypocalcemia in patients with osteoporosis: can you know who will get low? Osteoporos Int 2020; 31: 655-665.
32. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011; 96: 972-980.
33. Tsourdi E, Zillikens MC, Meier C, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2020.
34. Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med 2021; 10(1).
35. Noble J, McKenna M, Crowley R. Should denosumab treatment for osteoporosis be continued indefinitely? Ther Adv Endocrinol Metab 2021;12.
36. Inderjeeth CA, Inderjeeth AJ, Raymond WD. Medication selection and patient compliance in the clinical management of osteoporosis. Aust Fam Physician 2016; 45: 814-817.
37. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017; 377: 1417-1427.
38. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018; 391: 230-240.
39. Kim A, Girgis CM. Anabolic therapy: a new paradigm for osteoporosis management. Endocrinology Today 2021; 10(4): 20-25.
40. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532-1543.
41. Cosman F, Kendler DL, Langdahl BL, et al. Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int 2022; 33: 1243-1256.
42. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434-1441.
43. Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 2005; 26: 688-703.
44. Gilsenan A, Midkiff K, Harris D, Kellier-Steele N, McSorley D, Andrews E. Teriparatide did not increase adult osteosarcoma incidence in a 15-Year US postmarketing surveillance study. J Bone Miner Res 2021; 36: 244-251.