Subclinical hypothyroidism in older people: pathology or normal ageing?
Subclinical hypothyroidism is common in older individuals. Diagnosis is complicated by the upward shift in the normal thyroid stimulating hormone (TSH) range with ageing. Most people with elevated TSH levels spontaneously normalise on repeat testing. In people with persistent subclinical disease, most do not require treatment except in certain circumstances.
- The normal range for thyroid stimulating hormone (TSH) shifts upward with ageing.
- Subclinical hypothyroidism (TSH level above the reference range in combination with a normal free thyroxine level) is common in older individuals.
- Repeat testing is paramount as TSH levels normalise spontaneously in most people who have an initially elevated level.
- Classic symptoms of hypothyroidism overlap with ageing and chronic illness. Additionally, classic hypothyroid symptoms may be more subtle or absent in older people.
- Treatment with levothyroxine is warranted in people with overt hypothyroidism, regardless of age. In older people with persistent and asymptomatic subclinical disease, treatment is not recommended if TSH levels are below 10 mIU/L.
- Risks of overtreatment are greater in older people. Management decisions should factor in frailty, comorbidities and the burden of tests and medication use.
Pathology and ageing converge at the crossroads of perception; distinguishing between the two requires a discerning eye and a depth of understanding. Subclinical hypothyroidism is a biochemical diagnosis, characterised by an elevated thyroid stimulating hormone (TSH) level in combination with a free thyroxine (FT4) level within the population reference interval. Subclinical hypothyroidism is common within the general population, and the prevalence increases with age. In older individuals, the prevalence of subclinical hypothyroidism can be as high as 15%.1 As one in six people in Australia are aged 65 years and over, the implication is that up to 650,000 Australians may currently be affected by subclinical hypothyroidism.2
Diagnosis of thyroid dysfunction in older people is complicated by the expected increase in TSH level that occurs with age.3,4 Moreover, thyroid-associated signs and symptoms, such as fatigue, dry skin and depression, are common within older populations. Features can also overlap with symptoms arising from concurrent chronic illnesses, making it difficult to distinguish between the normal effects of ageing and true thyroid dysfunction. Growing evidence suggests that treatment of subclinical hypothyroidism in older people has no benefit in most situations, and may cause harm, particularly in those with milder cases.5 The risks associated with treatment are further compounded in older people due to the increased likelihood of overtreatment.5
This article outlines the nuances surrounding diagnosis and treatment of hypothyroidism in older people, with a focus on subclinical disease. It explores the effects of subclinical hypothyroidism in association with cardiovascular health and neurocognitive function and provides a practical approach to managing the older person with an elevated TSH level.
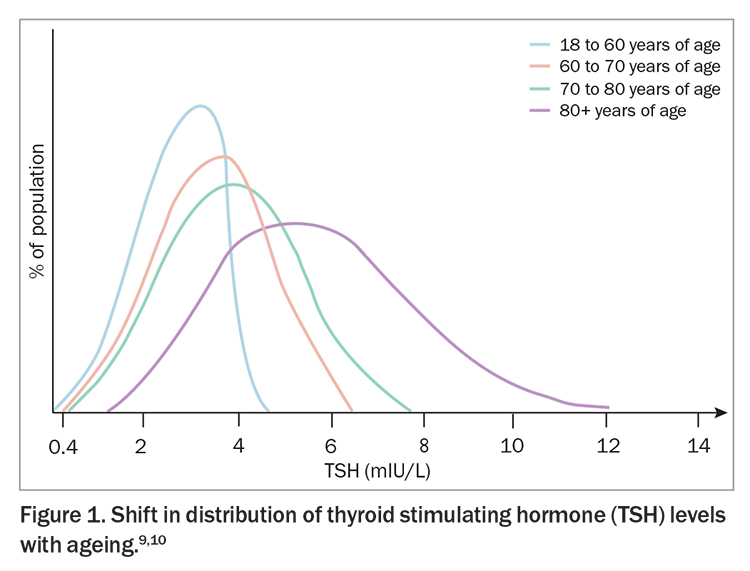
The TSH distribution shifts upward with age
The prevalence of overt hypothyroidism in older people is increased relative to the young healthy population. Diagnosis of overt hypothyroidism is made following measurement of an elevated TSH level combined with a FT4 level below the lower reference interval in a person with signs or symptoms of hypothyroidism. Overt hypothyroidism has been associated with poorer health outcomes, including in older people, and in almost all cases evidence supports treatment with levothyroxine in this setting.6
In contrast, subclinical hypothyroidism is more common and the prevalence may be as high as 15% for people aged 80 years and older.1 Prevalence is higher among women and in iodine-deficient individuals. The older person is broadly defined in most epidemiological studies as a person aged 65 years or older. Unlike overt hypothyroidism, treatment of subclinical hypothyroidism is controversial. Observational data suggest thyroid hormone replacement with levothyroxine does not improve outcomes and may be associated with harm, particularly in milder cases where TSH level remains below 10 mIU/L.7,8
Diagnosis of hypothyroidism is based on detection of a raised TSH level, with most upper limits of normal set at 4.5 mIU/L or below. As the TSH reference range is derived from a composite of all age groups in the general population, it fails to account for the normal upward shift in TSH that occurs with ageing (Figure 1).9,10 In a large sample of community dwelling adults, the upper limit of TSH was 3.5 mIU/L in adults aged 20 to 29 years, but increased to 7.5 mIU/L in those aged 80 years or older with no evidence of thyroid disease.11 Similar findings have been documented in an Australian population.3 Therefore, modified reference limits should be considered in the diagnosis of hypothyroidism in older patients.10
Causes of increased TSH levels in older people
Physiological causes
Multiple factors contribute to the expected increase in TSH level associated with healthy ageing. Thyroidal production of T4 and triiodothyronine (T3) decline by up to 30% from young adulthood to old age.5 The effects of reduced thyroid hormone production are partially offset by decreased clearance; however, compared with young healthy people, reduced peripheral conversion of T4 to T3 results in lower free T3 (FT3) levels and higher FT4:FT3 ratios among community dwelling older individuals.12 Additional factors may include downregulation of the hypothalamic–pituitary axis as an adaptive response to prevent excessive catabolism associated with the ageing process, decreased bioactivity of TSH, age-related thyroid fibrosis or relative iodine deficiency from reduced intestinal absorption and dietary salt restriction to manage other chronic illnesses.5,9,13
Pathogenic causes
The prevalence of thyroid autoantibodies increases with age, with positive thyroid peroxidase antibodies (TPOAb) present in up to 30% of people aged 70 years and older.11,14 Accordingly, Hashimoto’s thyroiditis is the most frequent cause of hypothyroidism in older people, followed closely by treatment of hyperthyroidism (e.g. thyroidectomy, radioactive iodine). Medications with potential to impact thyroid function are an important consideration in older patients. Medications in routine clinical use with the potential to impact thyroid function include glucocorticoids, antiepileptics, lithium, amiodarone and cancer treatments such as tyrosine kinase inhibitors and immune-checkpoint inhibitors. Supplements can also impact thyroid function or interfere with immunoassays used to measure TSH, FT4 and FT3 levels. Usually, thyroid dysfunction is reversible when the offending drug is withdrawn.9
Significance of subclinical hypothyroidism in older people
Overt hypothyroidism in older adults has been linked to an increased incidence of cardiovascular events, impaired cognitive function and mortality.9,15 The effect of subclinical hypothyroidism on clinical outcomes is less clear, particularly given the normal increase in TSH level that occurs with ageing. This has obvious implications for treatment and the main areas of focus have been on cardiovascular health and neurocognitive function.
Cardiovascular health
Observational data suggest that untreated subclinical hypothyroidism is not associated with an increased risk of coronary heart disease, cerebrovascular disease, cardiovascular mortality or all-cause mortality in older individuals with a TSH concentration below 7 mIU/L. As TSH increases above 7 mIU/L, the risk of coronary heart disease and heart failure appears to increase, particularly with TSH concentrations above 10 mIU/L.16-18
Neurocognitive function
Subclinical hypothyroidism has been linked to impaired cognitive function in younger adults, but in older adults the results are less consistent.19,20 A study of 1077 people aged 60 to 90 years found no association between TSH level and the risk of developing dementia.21 A separate prospective observational study of adults aged 85 to 89 years found no significant association between TSH level and disability in daily life, depressive symptoms or cognitive impairment over a mean follow up of 3.7 years.22 However, other observational studies of older persons have found impairments in cognitive performance associated with subclinical hypothyroidism.20
Does treatment help?
Few randomised trials have investigated the effect of levothyroxine replacement in older people with subclinical hypothyroidism. In those that have, disappointingly there was no benefit of levothyroxine therapy compared with placebo, although the mean TSH level at baseline in these trials was under 7 mIU/L.23,24 Additionally, these trials were underpowered to examine cardiovascular events or subgroups of patients with TSH levels above 7 mIU/L and, as such, our understanding of the effects of treatment of subclinical hypothyroidism in older individuals remains incomplete. Of note, there was no association between levothyroxine treatment and prespecified adverse events, including atrial fibrillation, heart failure and fracture. More voluminous, but less robust outcome data from cohort analyses have also shown no benefit from treatment with levothyroxine on cardiovascular events or all-cause mortality in older people with subclinical hypothyroidism.25,26
Clinical presentation and diagnostic considerations
Clinical features
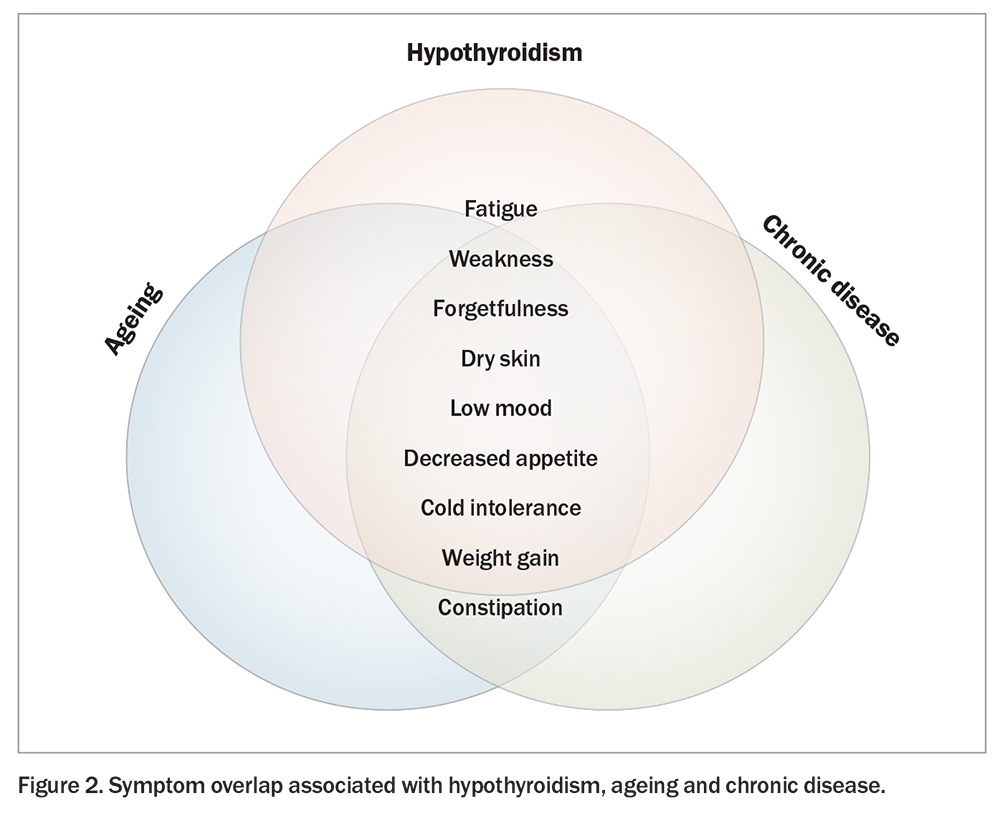
Symptoms of hypothyroidism are variable and nonspecific. Older people tend to report fewer symptoms overall compared with younger adults.27 Detection of hypothyroidism in older people is further complicated by the fact that symptoms may be more subtle and attributed to comorbid disorders (such as depression or anaemia) or to the ageing process itself (Figure 2). Younger adults typically report weight gain and cold intolerance, whereas older people mainly manifest with neuropsychiatric symptoms such as fatigue, weakness, memory loss or cognitive slowing.13 Typically, subclinical hypothyroidism is asymptomatic in older individuals. The degree of TSH elevation does not influence the likelihood or severity of symptoms and, as such, it is important to consider and exclude other potential causes before instituting thyroid hormone replacement.27
Considerations around testing
Screening for hypothyroidism in well older individuals is not routine or recommended.13 Testing should be restricted to people with signs or symptoms potentially attributable to hypothyroidism. Before checking thyroid function, clinicians should ensure the patient has had no recent illness or exposure to iodinated contrast that may cause a transient change in TSH levels.28 Independent factors that would support TSH screening include the presence of goitre, a history of thyroid surgery or neck irradiation, osteoporosis or minimal trauma fracture, atrial fibrillation or newly diagnosed cardiovascular disease.5
After an elevated TSH level has been detected, TSH testing should be repeated along with FT4 and TPOAb. In the setting of an elevated TSH level and low FT4 level, the diagnosis of overt hypothyroidism is made and treatment with levothyroxine is generally indicated. In the situation where the TSH level is elevated but FT4 level remains within the normal range, a diagnosis of subclinical hypothyroidism is made. Unlike overt hypothyroidism, treatment of subclinical hypothyroidism is not immediately indicated because, on repeat TSH testing after at least one month, about 60% of people spontaneously normalise and revert to euthyroidism.23,29 Antibody status is prognostically useful because people with positive TPOAb have a greater likelihood of progressing to overt hypothyroidism.30
Management
Compared with young healthy adults, special considerations apply to the management of overt and subclinical hypothyroidism in older people because of differences in metabolism, absorption, a higher likelihood of comorbidities, drug–drug interactions and potential risks associated with overtreatment.
General approach to management
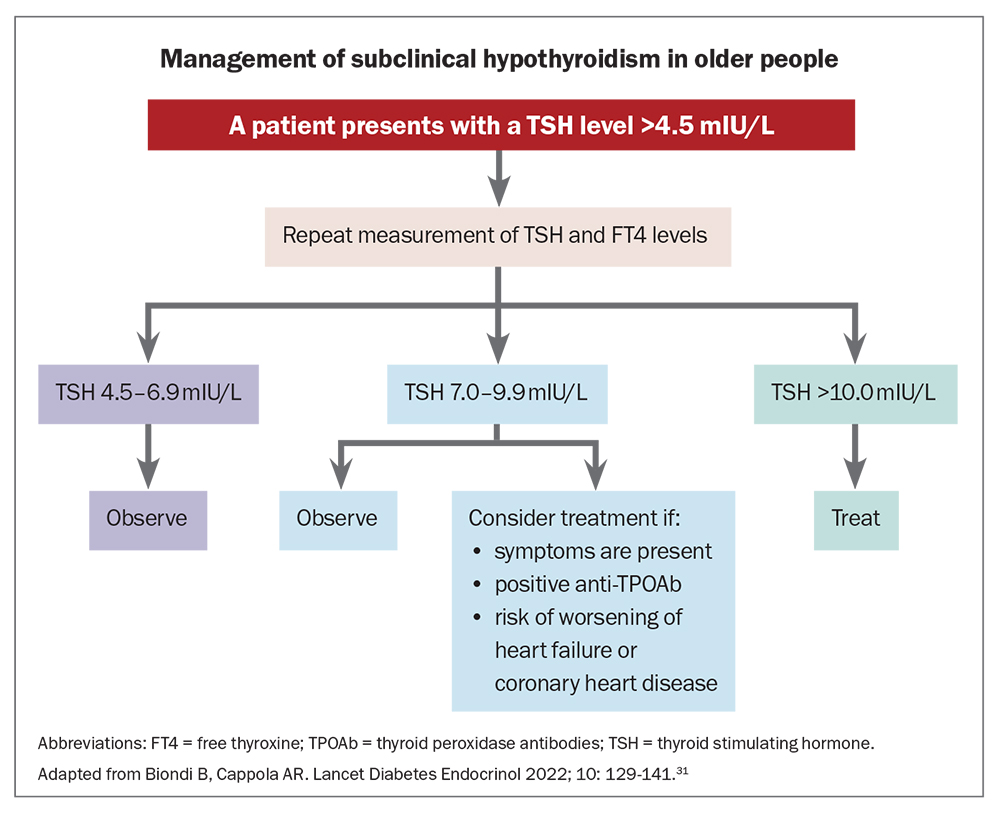
Thyroid hormone replacement in older individuals with overt hypothyroidism is recommended, whereas there is clinical equipoise around the treatment of subclinical hypothyroidism. Generally, levothyroxine is not recommended unless the TSH level is above 10 mIU/L, although treatment can be considered when the TSH level is between 5.0 and 9.9 mIU/L based on other influencing factors.7,8 These include relief of symptoms, avoiding progression to overt disease or to decrease the likelihood of adverse cardiovascular events in those at risk. In older patients with TSH levels below 10 mIU/L, monitoring of thyroid function is the preferred option. A suggested management algorithm for subclinical hypothyroidism in individuals over 65 years of age, stratified by TSH level, is shown in the Flowchart.31
Practical considerations
If considering treatment, practical issues in the older population influence whether potential benefits of levothyroxine outweigh the risks.32 These include:
- increased clinical appointments for periodic evaluation of thyroid function33
- taking a daily oral medication, adding to their tablet burden
- drug–drug interactions and administration difficulties (taken on an empty stomach, at least 30 minutes away from food and four hours away from medications containing calcium or iron)
- costs of medication and monitoring
- treatment dissatisfaction if levothyroxine fails to improve nonspecific symptoms7
- risks of overtreatment (see more below).
Levothyroxine prescription, TSH goals and the risks of overtreatment
Initial doses of levothyroxine in older individuals are typically lower than younger counterparts because of reduced drug metabolism and clearance, lower renal function and changes in body composition.5 Starting doses of 25 to 50 mcg daily are adequate, erring on the lower side if there is a history of arrhythmia or cardiovascular disease.34 In patients receiving levothyroxine, thyroid function tests should be measured predose or at least six hours postdose to avoid transient fluctuations in FT4 which occur following levothyroxine administration. TSH level should be repeated every six weeks after starting therapy until the TSH target has been achieved. Continued monitoring every six to 12 months should continue thereafter to ensure thyroid function remains stable and to avoid levothyroxine over-replacement and iatrogenic hyperthyroidism.
Iatrogenic hyperthyroidism, whether subclinical or overt, is associated with an increased risk of adverse events such as atrial fibrillation and osteoporosis, particularly in older and elderly individuals.5 Accordingly, it is reasonable to aim for a higher TSH target in treated older patients with overt or subclinical hypothyroidism (e.g. 4.0 to 7.0 mIU/L in people aged over 70 years), bearing in mind the use of age-adjusted normal ranges for TSH is based on expert opinion and has not yet been confirmed by high-quality randomised controlled trials.10
Conclusion
Subclinical hypothyroidism is common in older individuals. Current evidence suggests it has a limited impact on clinical outcomes. Special considerations in older people include a higher normal range TSH level, practical issues (e.g. cost of treatment, burden of testing and medication use, drug–drug interactions) and possible treatment-related harms. A watch-and-wait approach is preferred, except if the TSH level is above 10 mIU/L or above 7 mIU/L with caveats, in which case treatment should be initiated at a low dose aiming for a higher TSH target with close monitoring. ET
COMPETING INTERESTS: None.
References
1. Bensenor IM, Olmos RD, Lotufo PA. Hypothyroidism in the elderly: diagnosis and management. Clinical Interventions in Aging 2012; 7: 97-111.
2. Australian Institute of Health and Welfare (AIHW). Older Australians. Canberra: AIHW; 2023. Available online at https://www.aihw.gov.au/reports/older-people/older-australians/contents/summary (accessed February 2024).
3. Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol 2012; 97: 1554-1562.
4. Waring AC, Arnold AM, Newman AB, Bùžková P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab 2012; 97: 3944-3950.
5. Cappola AR, Auchus RJ, El-Hajj Fuleihan G, et al. Hormones and aging: an Endocrine Society Scientific Statement. J Clin Endocrinol Metab 2023: dgad225.
6. Nygaard B. Hypothyroidism (primary). BMJ Clin Evid 2014: 0605.
7. Ross DS. Treating hypothyroidism is not always easy: when to treat subclinical hypothyroidism, TSH goals in the elderly, and alternatives to levothyroxine monotherapy. J Intern Med 2022; 291: 128-140.
8. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2: 215-228.
9. Calsolaro V, Niccolai F, Pasqualetti G, et al. Overt and subclinical hypothyroidism in the elderly: when to treat? Front Endocrinol 2019; 10: 177.
10. Leng O, Razvi S. Hypothyroidism in the older population. Thyroid Res 2019; 12: 1-10.
11. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92: 4575-4582.
12. Duntas LH, Yen PM. Diagnosis and treatment of hypothyroidism in the elderly. Endocrine 2019; 66: 63-69.
13. Thiruvengadam S, Luthra P. Thyroid disorders in elderly: a comprehensive review. Dis Mon. 2021; 67: 101223.
14. Mariotti S, Barbesino G, Caturegli P, et al. Thyroid and other organ-specific autoantibodies in healthy ceritenarians. Lancet 1992; 339: 1506-1508.
15. Davis J, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinologica 2007; 32: 49-65.
16. Rodondi N, Bauer DC, Gussekloo J. Risk of coronary heart disease and mortality for adults with subclinical hypothyroidism. JAMA 2010; 304: 2481-2482.
17. Chaker L, Baumgartner C, Den Elzen WP, et al. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100: 2181-2191.
18. Gencer B, Collet T-H, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012; 126: 1040-1049.
19. Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28: 111-118.
20. Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med 2008; 168: 1514-1520.
21. de Jong FJ, den Heijer T, Visser TJ, et al. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 2006; 91: 2569-2573.
22. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA 2004; 292: 2591-2599.
23. Mooijaart SP, Du Puy RS, Stott DJ, et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA 2019; 322: 1977-1986.
24. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376: 2534-2544.
25. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172: 811-817.
26. Andersen MN, Olsen A-MS, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PloS One 2015; 10: e0129793.
27. Doucet J, Trivalle C, Chassagne P, et al. Does age play a role in clinical presentation of hypothyroidism? J Am Geriatr Soc 1994; 42: 984-986.
28. Franklyn JA. The thyroid–too much and too little across the ages. The consequences of subclinical thyroid dysfunction. Clin Endocrinol 2013; 78: 1-8.
29. Meyerovitch J, Rotman-Pikielny P, Sherf M, Battat E, Levy Y, Surks MI. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med 2007; 167: 1533-1538.
30. Huber G, Staub J-J, Meier C, Mitrache C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87: 3221-3226.
31. Biondi B, Cappola AR. Subclinical hypothyroidism in older individuals. Lancet Diabetes Endocrinol 2022; 10: 129-141.
32. Bekkering G, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 2019; 365: I2006.
33. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 2014; 174: 32-39.
34. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012; 22: 1200-1235.