Differentiating Hashimoto’s thyroiditis and Graves’ disease: a practical guide

Hashimoto’s thyroiditis and Graves’ disease are common autoimmune thyroid diseases that are often seen in primary care. They have some similar features and can be difficult to differentiate. GPs play an essential role in identification of the correct diagnosis for appropriate treatment and ongoing monitoring.
- Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) are the most common causes of hypothyroidism and hyperthyroidism, respectively.
- These conditions share common genetic associations and risk factors including female sex, excessive iodine intake, infection, stress and the postpartum period.
- In HT, autoantibodies are directed against thyroid peroxidase and thyroglobulin, whereas the thyroid-stimulating hormone receptor is the autoantibody target in GD.
- Patients with GD usually present with moderate-to-severe hyperthyroidism, goitre and extrathyroidal manifestations, and those with HT are often euthyroid or show subclinical to frank hypothyroidism.
- Diagnosis requires assessment of thyroid function and antibody status and may be supported by radiological findings.
- HT requires thyroxine replacement when hypothyroid, whereas GD is managed with antithyroid medications, radioiodine ablation or thyroidectomy.
Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) are the most common causes of thyroid dysfunction in Australia and are frequently encountered in primary care.1 The clinical presentation of thyroid dysfunction can be varied, and identifying the correct diagnosis is essential for appropriate treatment and ongoing monitoring.
This article covers the epidemiology and aetiology, pathophysiology, clinical presentation, investigation and treatment of HT and GD, as well as considerations for managing these conditions during pregnancy.
Epidemiology and aetiology
The most common autoimmune thyroid disease (AITD) is HT, also known as Hashimoto’s disease or chronic autoimmune lymphocytic thyroiditis. HT has a global prevalence of 7.5% and is the most common cause of hypothyroidism in people in iodine-sufficient areas.2,3 HT is more prevalent than GD, which affects about 0.5% of the population globally, although GD is still the main cause of hyperthyroidism.4-6 In Australia, it is estimated that the prevalences of overt and subclinical hypothyroidism are 0.5% and 5%, respectively, compared with 0.6% for both overt (0.3%) and subclinical (0.3%) hyperthyroidism.1
The aetiology of AITD is likely a combination of environmental factors and genetic susceptibility.7-10 Women are significantly more likely to be affected by AITD than men, with a sex ratio of 7:1 for HT and 5 to 10:1 for GD.4,11 Both GD and HT tend to peak around middle age (30 to 60 years of age).4,12 There are AITD clusters in families, and both HT and GD share some common genetic associations. In GD, the concordance rate in monozygotic twins is 20 to 35% and the sibling risk ratio has been reported to be as high as 11.6.10,13 In HT, the concordance rate in monozygotic twins has been reported to be as high as 55%, but only 3% in dizygotic twins.14
Risk factors for both HT and GD include female sex, excessive iodine intake, infection (although no specific infection is known to cause AITD), stress (immune suppression by non-antigen-specific mechanisms and possibly related to cortisol, followed by immune hyperactivity) and the postpartum period (reversal of immunosuppressive effects of pregnancy).15,16 Environmental radiation exposure has also been linked with HT, but study results vary.17,18 Risk factors specifically associated with GD include smoking, which is also associated with higher rates of Graves’ eye disease, and medications including lithium and alemtuzumab.19-22
AITDs are often associated with other autoimmune conditions because of shared genetic factors. A study reported that another autoimmune disorder, such as type 1 diabetes, Addison’s disease, coeliac disease, pernicious anaemia and rheumatoid arthritis, was found in 9.7% of patients with GD and in 14.3% of patients with HT.23
Pathophysiology
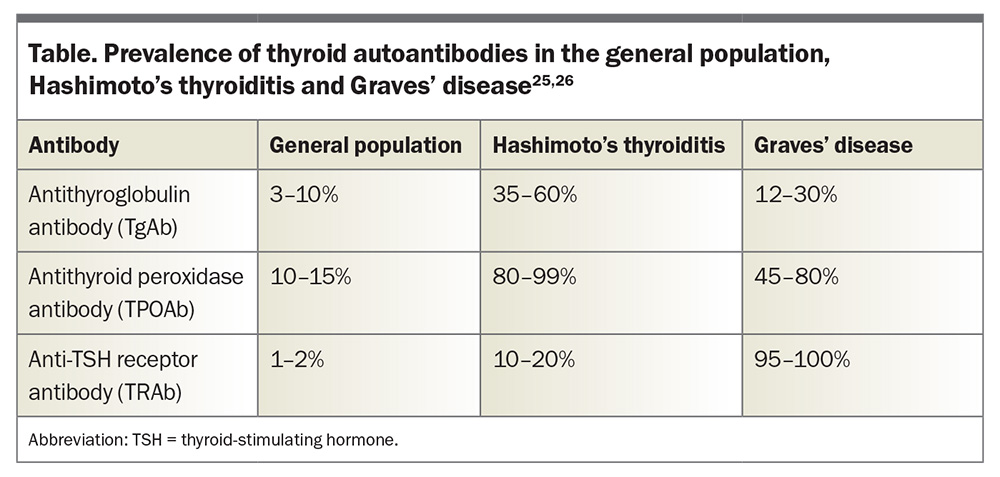
AITDs are characterised by the presence of circulating autoantibodies that are directed against various components of thyroid tissue and reactive T and B cells, reflecting a loss of immune tolerance.8,24 Autoantibodies can be found in up to 15% of the general population without thyroid dysfunction (Table).25,26
In HT, both lymphocytic infiltration and antibody-mediated destruction of thyroid tissue lead to gradual thyroid failure.3 Almost all patients with HT have antithyroid peroxidase antibodies (TPOAb), which may contribute to immunopathology through complement fixing.25 Antithyroglobulin antibodies (TgAb) are also present in most patients with HT; however, they do not appear to play a role in immunopathology.25
In contrast, GD is caused by stimulating thyroid-stimulating hormone (TSH) receptor antibodies (TRAb), which replicate the action of TSH and cause increased thyroid hormone production and goitre due to follicular hypertrophy and hyperplasia.4 The TSH receptor can also be found on other tissues such as fibroblasts and adipocytes, accounting for various extrathyroidal manifestations of GD such as orbitopathy.
Although HT and GD are distinct disorders, they lie on a spectrum of thyroid autoimmunity. TPOAb and TgAb are more common in HT but are also often found in GD (Table). TRAb are more common in GD, but it is possible for patients with HT to have mildly positive TRAb too, and these are often neutral or blocking antibodies.25-29 In patients who are euthyroid, the differentiation between GD and HT based on the presence of thyroid antibodies alone can be difficult.
Clinical presentation
Hashimoto’s thyroiditis
Patients with HT are often euthyroid and may be asymptomatic. It has been reported that 20% of patients will present with symptoms and signs of mild hypothyroidism.30 However, because of the progressive thyroid dysfunction in HT, hypothyroidism more commonly develops over several years. Symptoms can be nonspecific and include fatigue, cold intolerance, weight gain, constipation, dry skin and menstrual irregularity.
The thyroid can enlarge gradually, which the patient may notice as neck fullness, although some patients will have thyroid atrophy. It is rare that the goitre in HT will be significantly large enough to cause compressive symptoms. Gross thyroid nodularity is not suggestive of HT, but there may be co-occurrence of a multinodular goitre and HT.12 Often the remainder of the physical examination is normal.
Occasionally, a patient with HT may initially present with hyperthyroidism caused by transient thyroiditis. Very rarely, hyperthyroidism may be caused by stimulating TRAb, similar to in GD, followed by hypothyroidism, termed Hashitoxicosis.31
Graves’ disease
Patients with GD often present with moderate- to-severe hyperthyroidism. Patients may report heat intolerance, sweating, palpitations, weight loss, increased bowel frequency, tremor, anxiety, insomnia, or fatigue and generalised weakness. Women may develop menstrual irregularity and, occasionally, men may present with gynaecomastia or erectile dysfunction.32 Older patients may present with predominantly cardiovascular manifestations such as new atrial fibrillation or exertional dyspnoea.33
Physical examination may reveal a diffuse non-nodular goitre, and orbitopathy is clinically apparent in 30 to 50% of patients with GD.4 Lid retraction and lid lag are secondary to sympathetic hyperactivity and can be seen in any form of hyperthyroidism, whereas features specific to GD are exophthalmos, periorbital and conjunctival oedema, and restricted eye movements.4,34 Other signs of GD include tachycardia, tremor, proximal muscle weakness, hyperreflexia, warm skin, and thin or fine hair. An infiltrative dermopathy, termed pretibial myxoedema, may rarely be seen in GD.35 Historically, GD was diagnosed clinically (i.e. the triad of hyperthyroidism, a diffuse goitre and an extrathyroidal manifestation such as orbitopathy), but the diagnosis is now largely supported by investigations.
Investigations
Pathology
If thyroid disease is suspected clinically, the most sensitive initial test is measurement of the TSH level. Some laboratories will add on measurement of free thyroxine (FT4) and free triiodothyronine (FT3) levels if the TSH level is abnormal, otherwise an add-on request is required. However, if clinical suspicion is high then measurement of FT4 and FT3 levels can be requested initially.
In patients with overt (high TSH, low FT4 levels) or subclinical (high TSH, normal FT4 levels) hypothyroidism, measurement of TPOAb and TgAb levels can help distinguish HT from other causes. In patients with subclinical hypothyroidism, there is about a 5% risk of developing overt hypothyroidism per year.36,37 Predictive factors for future development of hypothyroidism include the presence of goitre and TgAb at presentation, and progressive increases in TSH and TPOAb levels.12 Patients who are positive for TPOAb but have normal thyroid function are more likely to develop hypothyroidism than those who are antibody-negative.38
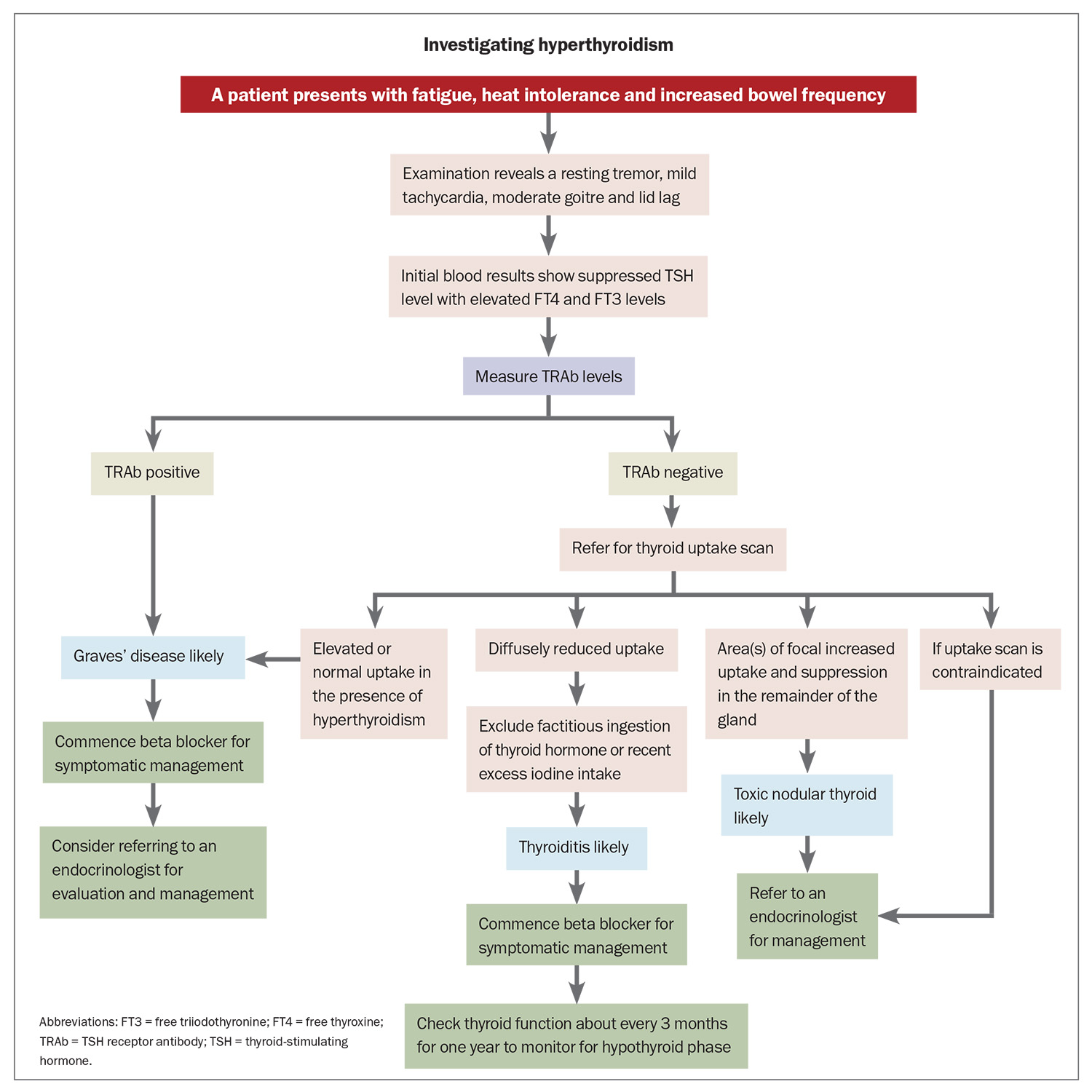
In patients with overt (low TSH, high FT4 levels) or subclinical (low TSH, normal FT4 levels) hyperthyroidism, a test for TRAb should be ordered to investigate for GD (Flowchart), noting that it can be negative in the early stages or in mild cases. The FT4/FT3 ratio can also be useful because in GD there is a higher proportion of FT3 secretion from the thyroid and increased conversion of FT4 to FT3 in the periphery.4 One study found that an FT3/FT4 ratio of greater than 0.3 SI units was suggestive of GD.39 Conversely, FT3 level is not as high in other causes of hyperthyroidism such as thyroiditis or toxic nodular goitre.
Thyroid uptake scan
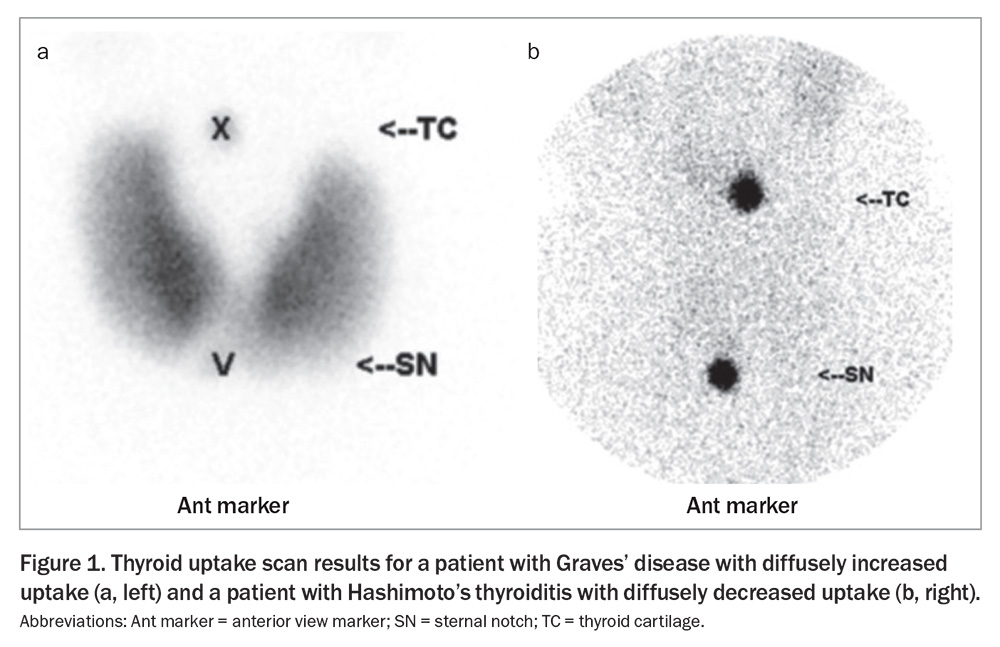
A thyroid uptake scan can help distinguish the cause of hyperthyroidism. The radioactive tracer uptake is usually elevated in patients with GD; it is normal or high in toxic nodular goitre and near zero in patients with thyroiditis, factitious ingestion of thyroid hormone or recent excess iodine intake (Figure 1).40 A single toxic adenoma generally shows focal uptake in the adenoma with suppressed uptake in the surrounding and contralateral thyroid tissue.40 It is recommended to wait at least six weeks after intravenous contrast before performing an uptake scan as the iodine load may suppress uptake.
Thyroid ultrasound
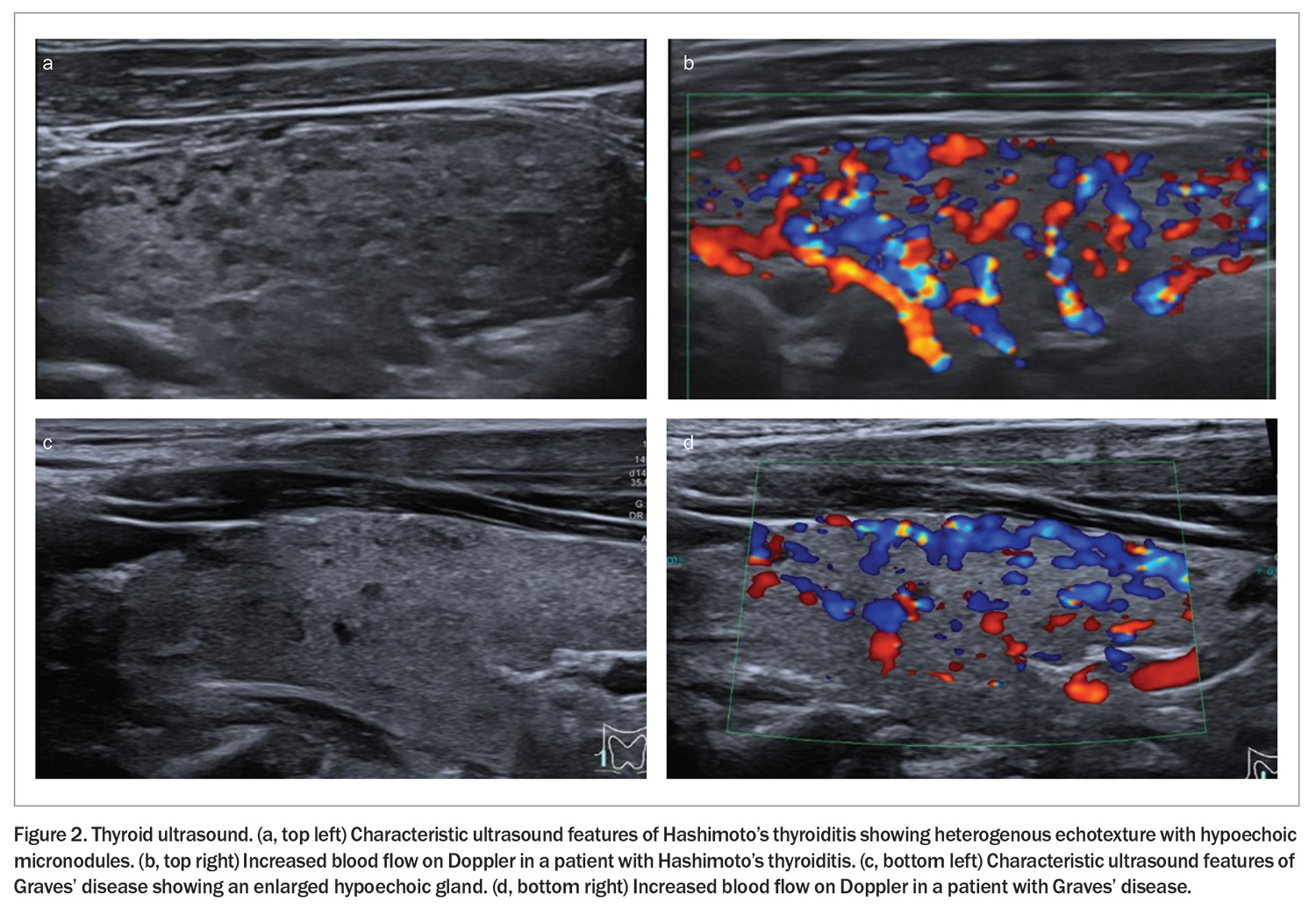
A thyroid ultrasound should not be routinely ordered in hypothyroidism unless there is a palpable abnormality such as a nodule or goitre.41 If performed in HT, the ultrasound will typically show a mildly enlarged gland with hypoechoic, diffusely heterogenous echotexture with hypoechoic micronodules, and there may be increased thyroidal blood flow on the Doppler (Figure 2). Fine-needle aspiration is not required in HT unless a discrete nodule is being investigated.12,42
In patients with hyperthyroidism, a thyroid ultrasound can be useful when radioactive iodine is contraindicated (such as in pregnancy). Ultrasound with colour flow Doppler can distinguish thyroid hyperactivity (increased flow) from thyroiditis.43 As increased blood flow can be seen in both HT and GD, this alone cannot reliably distinguish between the two conditions.
Treatment
Hashimoto’s thyroiditis
Euthyroid patients with HT do not require treatment. If hypothyroidism is present, then thyroid hormone replacement therapy is usually indicated. Older patients (over 60 years of age) or those with cardiac history should have a lower dose, such as 25 to 50 mcg per day initially.44 The goal of therapy is symptom resolution (if present) and to normalise thyroid function, noting that TSH levels can take months to improve. Thyroid antibodies do not need to be re-measured while the patient is receiving therapy as this will not affect management. Once thyroxine treatment is initiated, it is required indefinitely in most patients.12 Thyroid hormone replacement therapy often reduces the size of the goitre after several months of treatment.12
For subclinical hypothyroidism, it is recommended to start thyroxine in patients with a TSH level of greater than 10 mIU/L.1 If the TSH level is between the upper limit of normal and 10 mIU/L, it can often normalise on repeat testing without treatment and does so in over 50% of cases.45 It should also be noted that the TSH level may increase with age.46,47 The decision to treat depends on several factors, including the presence of symptoms, the age of the patient and the presence of comorbidities such as cardiovascular disease.
For those rare patients with HT who present with hyperthyroidism, management is focused on symptom control with a beta blocker if required. Thyroid function should be monitored to screen for a hypothyroid phase. After recovery, it is possible for patients to have subsequent episodes of thyroiditis, and there is an overall increased risk of hypothyroidism.
It is important to note in patients with adrenal insufficiency and hypothyroidism that replacement of thyroid hormone prior to steroid replacement can precipitate an adrenal crisis. If clinical suspicion of adrenal insufficiency is high, then it is recommended to check the patient’s morning cortisol before initiating thyroxine replacement.
Graves’ disease
Beta-adrenergic blockade is recommended in patients with symptomatic thyrotoxicosis.40 Once the diagnosis of GD is confirmed, alongside beta-adrenergic blockade, the options for treatment include medical management with antithyroid medication, radioiodine and surgery.4 Referral to an endocrinologist is generally recommended.
Carbimazole is the antithyroid medication of choice because of its longer intrathyroidal half-life and more favourable side effect profile compared with propylthiouracil (PTU).48,49 Patients should be counselled about the rare risk of agranulocytosis (0.1 to 0.3%) and hepatotoxicity.48 Of those treated with antithyroid medication, the average rate of remission (normal TSH level after medication cessation for more than 12 months) is 30 to 50%; however, over 50% of patients relapse.48 Factors associated with lower remission rates include male sex, older age, persistently elevated TRAb levels and patients with more active disease (i.e. higher thyroid hormone or TRAb levels, large goitre).50 Some patients may prefer long-term medical management and others may prefer definitive treatment.
Definitive treatment options for GD include radioiodine and surgery, which results in permanent hypothyroidism. There is a risk of worsening thyroid eye disease and a rare risk of radiation thyroiditis from radioiodine treatment, whereas total thyroidectomy comes with surgical risks including iatrogenic hypoparathyroidism and recurrent laryngeal nerve damage.
A discussion of specific treatment for Graves’ eye disease is beyond the scope of this article; however, it varies from selenium in mild cases to intravenous glucocorticoids or immunosuppressive therapies in moderate- to-severe cases and rarely orbital decompression surgery.
Considerations in pregnancy
It is recommended that women with subclinical hypothyroidism who are planning pregnancy or with a history of infertility are commenced on thyroxine therapy.51 For women who are already receiving thyroxine therapy pre-pregnancy, the dose will generally need to be increased by at least 30% upon confirmation of pregnancy, targeting a TSH level of less than 2.5 mIU/L.51 Women who are euthyroid with positive TPOAb are at a higher risk of developing subclinical hypothyroidism in pregnancy, although current American Thyroid Association guidelines recommend initiating therapy only if the TSH level is at least 4.0 mIU/L.51 The presence of TPOAb also increases the risk of postpartum thyroiditis.
For women with GD, contraception is advised until hyperthyroidism is controlled.52 In those wanting to pursue pregnancy, thyroidectomy could be considered for definite therapy as radioactive iodine is contraindicated from six months preconception until completion of breastfeeding.52 Antithyroid medications cross the placenta and can affect fetal thyroid function.53,54 In the first trimester, PTU is preferred because of the rare association of carbimazole with fetal defects.4 TRAb can also cross the placenta and cause hyperthyroidism in the fetus, and it is recommended that serial scans are performed to monitor fetal heart rate and to check for fetal goitre in mothers with elevated TRAb levels. GD tends to be more severe in the first trimester and improves as the pregnancy progresses but may flare during postpartum.4,53
Conclusion
GD and HT are both common autoimmune conditions in the general population. Although they lead to two different spectra of thyroid dysfunction, they share some common risk factors and can be associated with other autoimmune conditions. HT is usually permanent and managed with thyroxine replacement once the patient is hypothyroid, whereas GD is characterised by periods of remission and relapse and can be cured with definitive therapy. GPs play an essential role in recognising the thyroid dysfunction and performing ongoing monitoring of thyroid function in patients with autoimmune disease of the thyroid to appropriately titrate therapy and screen for complications. ET
COMPETING INTERESTS: None.
References
1. Walsh JP. Managing thyroid disease in general practice. Med J Aust 2016; 205: 179-189.
2. Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: a systematic review and meta-analysis. Front Public Health 2022; 10: 1020709.
3. Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev 1994; 15: 788-830.
4. Brent G. Graves’ disease. N Engl J Med 2008; 358: 2594-2605.
5. Weetman AP. Graves’ disease. N Engl J Med 2000; 343: 1236-1248.
6. Cooper DS. Hyperthyroidism. Lancet 2003; 362: 459-468.
7. Stenszky V, Kozma L, Balázs C, Rochlitz S, Bear JC, Farid NR. The genetics of Graves’ disease: HLA and disease susceptibility. J Clin Endocrinol Metab 1985; 61: 735-740.
8. Tomer Y, Ban Y, Concepcion E, et al. Common and unique susceptibility loci in Graves and Hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet 2003; 73: 736-747.
9. Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr Rev 2003; 24: 694.
10. Grixti L, Lane LC, Pearce SH. The genetics of Graves’ disease. Rev Endocr Metab Disord 2024; 25: 203-214.
11. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87: 489-499.
12. Akamizu T, Amino N. Hashimoto’s thyroiditis. [Updated 2017] In: Feingold KR, Anawalt B, Blackman MR, et al., ed. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK285557/ (accessed May 2025).
13. Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid 2003; 13: 761-764.
14. Brix TH, Hegedüs L, Gardas A, Banga JP, Nielsen CH. Monozygotic twin pairs discordant for Hashimoto’s thyroiditis share a high proportion of thyroid peroxidase autoantibodies to the immunodominant region A. Further evidence for genetic transmission of epitopic ‘fingerprints’. Autoimmunity 2011; 44: 188-194.
15. Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev 1993; 14: 107-120.
16. Bendtzen K, Buschard K, Diamant M, et al. Possible role of IL-1, TNF-alpha, and IL-6 in insulin-dependent diabetes mellitus and autoimmune thyroid disease. Thyroid Cell Group. Lymphokine Res 1989; 8: 335-340.
17. Völzke H, Werner A, Wallaschofski H, Horn T, Svenson M. Occupational exposure to ionizing radiation is associated with autoimmune thyroid disease. J Clin Endocrinol Metab 2005; 90: 4587-4592.
18. Tronko MD, Brenner AV, Olijnyk VA, et al. Autoimmune thyroiditis and exposure to iodine 131 in the Ukrainian cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: results from the first screening cycle (1998-2000). J Clin Endocrinol Metab 2006; 91: 4344-4351.
19. Prummel MF, Wiersinga WM. Smoking and risk of Graves’ disease. JAMA 1993; 269: 479-482.
20. Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med 2009; 360: 994-1001.
21. McDermott MT, Burman KD, Hofeldt FD, Kidd GS. Lithium-associated thyrotoxicosis. Am J Med 1986; 80: 1245-1248.
22. Daniels GH, Vladic A, Brinar V, et al. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocrinol Metab 2014; 99: 80-89.
23. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med 2010; 123: 183.e1-9.
24. Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun 2009; 32: 231-239.
25. Saravanan P, Dayan CM. Thyroid autoantibodies. Endocrinol Metab Clin North Am. 2001; 30: 315-337.
26. Vargas-Uricoechea H, Nogueira JP, Pinzón-Fernández MV, Schwarzstein D. The usefulness of thyroid antibodies in the diagnostic approach to autoimmune thyroid disease. Antibodies (Basel). 2023; 12: 48.
27. Konishi J, Iida Y, Kasagi K, et al. Primary myxedema with thyrotrophin-binding inhibitor immunoglobulins. Clinical and laboratory findings in 15 patients. Ann Intern Med 1985; 103: 26-31.
28. Kraiem Z, Lahat N, Glaser B, Baron E, Sadeh O, Sheinfeld M. Thyrotrophin receptor blocking antibodies: incidence, characterization and in-vitro synthesis. Clin Endocrinol (Oxf) 1987; 27: 409-421.
29. Kraiem Z, Cho BY, Sadeh O, Shong MH, Pickerill P, Weetman AP. The IgG subclass distribution of TSH receptor blocking antibodies in primary hypothyroidism. Clin Endocrinol (Oxf) 1992; 37: 135-140.
30. Gordin A, Saarinen P, Pelkonen A, Lamberg BA. Serum thyroglobulin and the response to thyrotropin releasing hormone in symptomless autoimmune thyroiditis and in borderline and overt hypothyroidism. Acta Endocrinol (Copenh) 1974; 75: 274-285.
31. Fatourechi V, McConahey WM, Woolner LB. Hyperthyroidism associated with histologic Hashimoto’s thyroiditis. Mayo Clin Proc 1971; 46: 682-689.
32. Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab 2005; 90: 6472-6479.
33. Boelaert K, Torlinska B, Holder RL, Franklyn JA. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study. J Clin Endocrinol Metab 2010; 95: 2715-2726.
34. Khoo TK, Bahn RS. Pathogenesis of Graves’ ophthalmopathy: the role of autoantibodies. Thyroid 2007; 17: 1013-1018.
35. Schwartz KM, Fatourechi V, Ahmed DDF, Pond GR. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab 2002; 87: 438-446.
36. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995; 43: 55-68.
37. Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87: 3221-3226.
38. Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O’Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab 2010; 95: 1095-1104.
39. Izumi Y, Hidaka Y, Tada H, et al. Simple and practical parameters for differentiation between destruction-induced thyrotoxicosis and Graves’ thyrotoxicosis. Clin Endocrinol (Oxf) 2002; 57: 51-58.
40. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016; 26: 1343-1421.
41. Endocrine Society of Australia. Top 5 low-value practices and interventions. Sydney: Royal Australasian College of Physicians; 2015. Available online at: http://evolve.edu.au/published-lists/esa (accessed May 2025).
42. Anderson L, Middleton W, Teefey S, et al. Hashimoto thyroiditis: part 1, sonographic analysis of the nodular form of Hashimoto thyroiditis. AJR Am J Roentgenol 2010; 195: 208-215.
43. Bogazzi F, Vitti P. Could improved ultrasound and power Doppler replace thyroidal radioiodine uptake to assess thyroid disease? Nat Clin Pract Endocrinol Metab 2008; 4: 70-71.
44. Gavigan C, Abbey EJ, McGready J, Simonsick EM, Mammen JS. Levothyroxine dosing in older adults: recommendations derived from the Baltimore Longitudinal Study of Aging. Endocr Pract 2023; 29: 612-617.
45. Meyerovitch J, Rotman-Pikielny P, Sherf M, Battat E, Levy Y, Surks MI. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med 2007; 167: 1533-1538.
46. Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7: 232-239.
47. Kahapola-Arachchige KM, Hadlow N, Wardrop R, Lim EM, Walsh JP. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin Endocrinol (Oxf) 2012; 77: 773-779.
48. Cooper DS. Antithyroid drugs. N Engl J Med 2005; 352: 905-917.
49. Nakamura H, Noh JY, Itoh K, Fukata S, Myauchi A, Hamada N. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab 2007; 92: 2157-2162.
50. Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SCL, Franklyn JA. Age and gender predict the outcome of treatment for Graves’ hyperthyroidism. J Clin Endocrinol Metab 2000; 85: 1038-1042.
51. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27: 315-389.
52. Ashkar C, Sztal-Mazer S, Topliss DJ. How to manage Graves’ disease in women of childbearing potential. Clin Endocrinol (Oxf) 2023; 98: 643-648.
53. Chan GW, Mandel SJ. Therapy insight: management of Graves’ disease during pregnancy. Nat Clin Pract Endocrinol Metab 2007; 3: 470-478.
54. Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2007; 92: Suppl:S1-S47.