Thyroiditis – differentiating the cause
The varied causes of thyroiditis can be challenging to differentiate. A working knowledge of the clinical features associated with each subtype is required. Reaching the correct diagnosis relies on informed assessment, focused examination and appropriate investigation.
- Thyroiditis is a general term for diverse diagnoses characterised by inflammation of the thyroid and is classified as painful or painless thyroiditis. Autoimmune and drug induced thyroiditis are the main causes of painless thyroiditis. Painful thyroiditis is usually due to subacute thyroiditis but rarer forms should be considered.
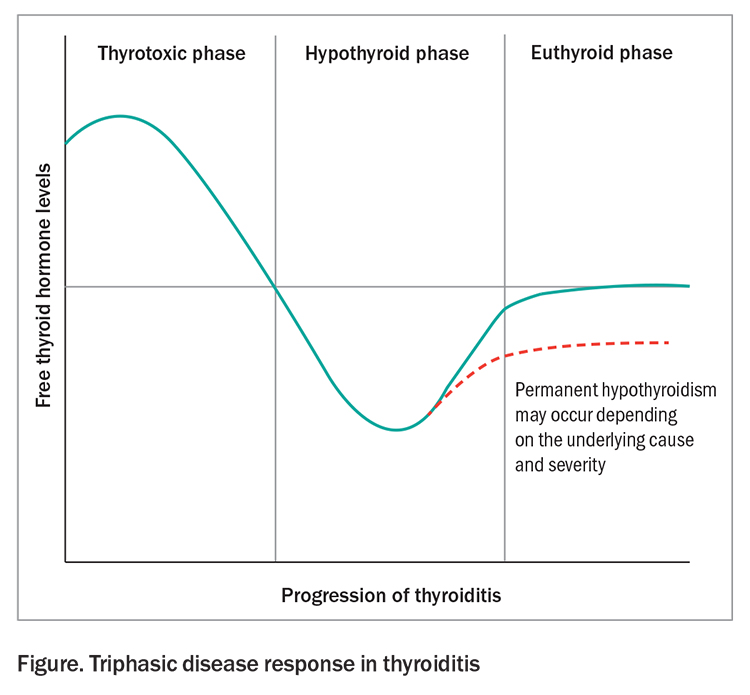
- Common features include the classic triphasic pattern of: thyroid dysfunction of the thyrotoxic phase, due to release of preformed thyroid hormones from damaged follicles; followed by hypothyroidism secondary to depleted stores; and then a return to euthyroid state from recovery, which can take six to 12 months.
- General principles of management include symptomatic treatment for pain (if present) with anti-inflammatory agents, beta blockers for management of symptoms in the thyrotoxic phase and levothyroxine for management of symptoms in cases of hypothyroidism.
- Thioamides are not effective in thyroiditis, except in some forms of drug induced thyroiditis. Graves’ disease is an important differential diagnosis in the thyrotoxic phase of thyroiditis and requires treatment with thioamides.
Thyroiditis is characterised by inflammation of the thyroid gland and results in damage to its follicular cells. The classic triphasic disease response includes the initial hyperthyroid phase, characterised by the release of preformed thyroid hormones from damaged follicles, followed by hypothyroidism secondary to depleted stores with a subsequent eventual recovery to a euthyroid state six to 12 months later (Figure).
The myriad diagnoses of thyroiditis are generally classified according to their associated symptoms of pain or painlessness. The differential diagnoses of painless thyroiditis include Hashimoto’s thyroiditis, postpartum thyroiditis, silent thyroiditis and drug induced thyroiditis. Neck pain with thyroiditis is commonly due to subacute thyroiditis, but rarer causes should be excluded. This article describes the causes of thyroiditis and provides a guide for its evaluation and management in primary care.
Clinical evaluation
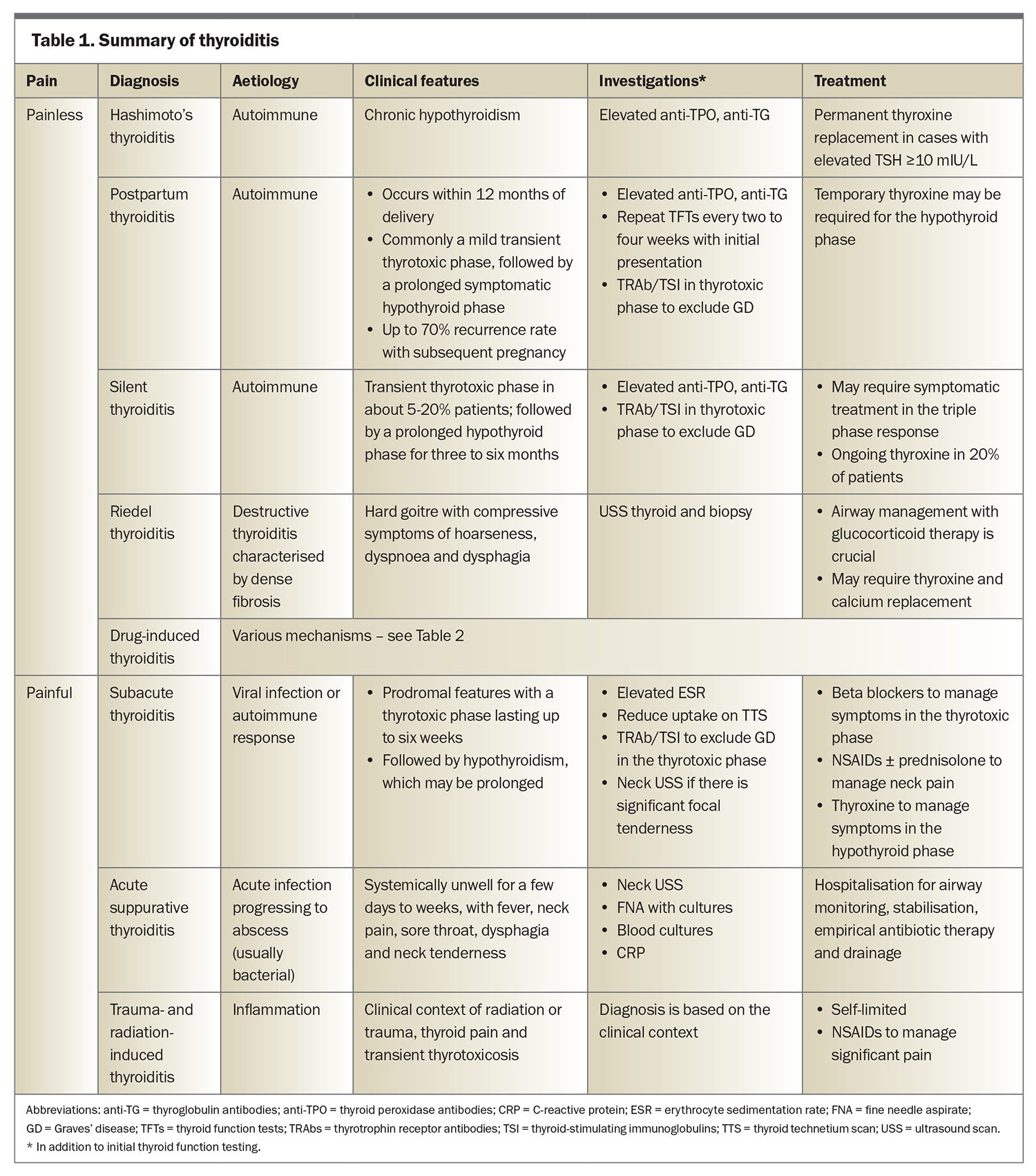
The key determinants in the clinical presentation are the presence of neck pain and thyroid dysfunction (Table 1). Provisional diagnoses are informed by the clinical context and the rapidity of onset and evolution of symptoms. In addition to thyroid function tests (TFTs) and depending on the clinical scenario, thyroid antibody levels, erythrocyte sedimentation rate (ESR), thyroid technetium scanning and thyroid ultrasound scanning may help as confirmatory investigations.
Neck pain
Subacute thyroiditis presents with neck pain in more than 90% of patients.1 Rare causes of neck pain with thyroid dysfunction include suppurative bacterial thyroiditis, trauma and radiation induced thyroiditis. Although not a cause of thyroiditis, spontaneous haemorrhage of a thyroid nodule can result in a similarly painful, palpable mass in the neck.2
Thyroid dysfunction
Thyrotoxicosis
Thyrotoxicosis is seen in the initial course of the triphasic response in both painful and painless forms of thyroiditis. Symptoms of thyrotoxicosis vary in severity and duration, with palpitations, diaphoresis, weight loss and anxiety as the predominant features. Examination findings may include tachycardia, fine tremor and brisk reflexes. TFTs usually indicate suppressed thyroid-stimulating hormone (TSH) with normal or elevated free thyroid hormone levels. Thyroid technetium scans provide a definitive diagnosis of thyroiditis, with findings of low uptake of technetium by the thyroid during the thyrotoxic phase. Graves’ disease and autonomous nodules are the main differential diagnoses to consider in patients with thyrotoxicosis and can be confirmed with findings of normal to increased uptake on thyroid technetium scans with or without positive thyrotropin receptor antibodies.
Hypothyroidism
Temporary hypothyroidism follows the thyrotoxic phase and can have a prolonged duration of up to six months. Euthyroid state without a hypothyroid state may occur in cases of mild thyroiditis. All types of thyroiditis may progress to permanent hypothyroidism, which occurs most commonly in patients with high levels of thyroid antibodies and an associated phase of severe hypothyroidism.
Patients experiencing severe hypothyroidism are more likely to present with classical symptoms, such as lethargy, weight gain, cold intolerance, decreased bowel movements, muscle aches, depression, menorrhagia, and dry skin and hair. Clinical signs of hypothyroidism include bradycardia, decreased reflexes, coarse skin and hair and fluid retention. TFTs typically show an elevated TSH level with a low free thyroxine level.
Treatment of thyroiditis
Symptoms of thyroiditis are typically mild and transient. Beta blockers can be used to manage symptoms of severe thyrotoxicosis; antithyroid drugs are ineffective, as there is no excess thyroid hormone production except in certain types of drug induced thyroiditis. Levothyroxine should be considered in symptomatic patients with a prolonged hypothyroid phase, with reassessment for ongoing use every six to 12 months. NSAIDs and corticosteroids can be used to manage symptoms of neck pain.
Specific causes of thyroiditis
Hashimoto’s thyroiditis
Hashimoto’s thyroiditis (HT) is the most common autoimmune thyroid disorder. It is commonly associated with chronic hypothyroidism but can instead follow the triphasic pattern of thyroiditis. HT is characterised by lymphocytic infiltration of the thyroid gland due to loss of immune tolerance, with a resulting elevation of thyroid antibodies (thyroid peroxidase antibodies, thyroglobulin antibodies) and eventual thyroid failure.
Around 10 to 20% of the Australian population has evidence of thyroid autoimmunity. Prevalence increases with age and 1 to 2% of patients experience features of overt hypothyroidism.3 The likelihood of overt hypothyroidism is markedly elevated in patients with elevated TSH and thyroid antibody levels.4
Genetic susceptibility and environmental risk factors (especially higher iodine intake) are predisposing factors for HT. Patients with HT have an increased risk of other organ-specific or systemic autoimmunity. Polyglandular autoimmune syndromes are characterised by more than one immune-mediated endocrinopathy, with a high prevalence of type 1 diabetes, Addison’s disease, hypogonadism, autoimmune thyroid disease, vitiligo and alopecia.5 Higher prevalence of systemic autoimmune disorders (e.g. rheumatoid arthritis) has been noted in patients with autoimmune thyroiditis.6
HT is a well-recognised risk factor for the development of thyroid lymphoma, with a 40 to 80 times greater risk compared with the general population; however, less than 1% of patients with HT develop thyroid lymphoma.7 Clinical presentations are dramatic with a sudden increase in goitre and airway compromise over a matter of weeks.
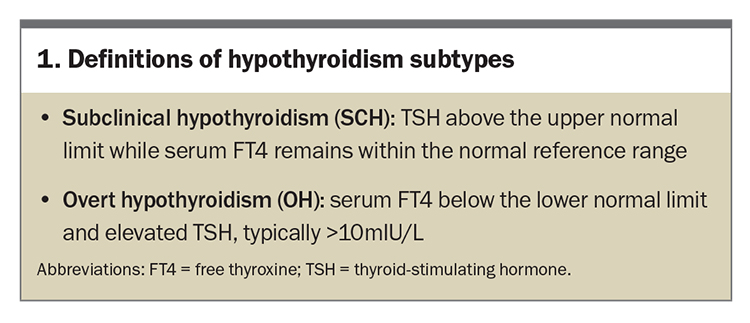
Serum TSH levels and thyroid peroxidase (TPO) antibody levels are required for the diagnosis of Hashimoto’s thyroid disease; thyroid technetium scanning, thyroid ultrasound scanning and thyroid biopsies are not routinely indicated. Initially, subclinical hypothyroidism (SCH) occurs and further progression of thyroiditis results in overt hypothyroidism (Box 1).
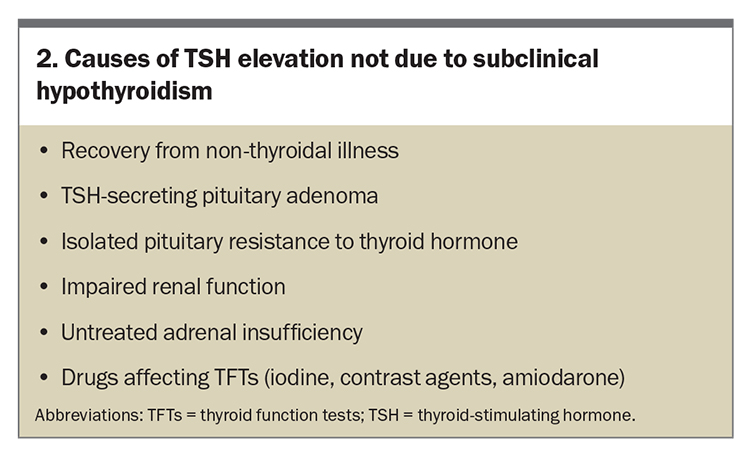
Other potential causes of elevated TSH levels should be considered, ranging from early thyroid failure to transient increases due to nonthyroidal illness, with reassessment of TSH levels after two to three months (Box 2). Age- and race-specific TSH distribution and reference limits may need to be considered in clinical practice. A shift in TSH distribution to higher concentrations with age in healthy elderly patients has been demonstrated.8
Most guidelines recommend levothyroxine for the treatment of overt hypothyroidism, which is associated with an increased risk of hypothyroid symptoms and cardiovascular events.9 A starting thyroxine dosage of 1.6 mcg/kg/day is recommended, with subsequent changes made every six to 12 weeks to maintain TSH levels within the reference range. For elderly patients and patients with significant cardiac disease, a lower initial dosage of 25 to 50 mcg/day with gentle titration is recommended. More frequent adjustment of thyroxine is not recommended, as thyroxine has a long half-life and a steady-state is not achieved until six weeks have lapsed.
In euthyroid patients with HT, annual TFTs are recommended due to the risk of disease progression to hypothyroidism. Annually, 2 to 6% of patients with SCH progress to overt hypothyroidism, with a higher risk in women, patients with elevated TSH levels, and patients with a higher TPO antibody titre.10,11
Levothyroxine confers no benefit in managing symptoms of hypothyroidism in older people with SCH.12 Although treatment of SCH has been associated with improvement of left ventricular ejection fraction, dyslipidaemia and carotid intima media thickness, a reduction in cardiovascular disease risk has not been consistently shown.13-15 A retrospective study showed treatment of SCH was associated with fewer ischaemic heart disease events in younger individuals, but this was not evident in older people.16,17 Treatment is therefore not recommended in patients with SCH aged 70 years and over. Treatment can be considered in younger patients with SCH (even when TSH levels are <10 mIU/L), particularly if patients are experiencing hypothyroid symptoms and cardiovascular risks are present.18,19
Treatment is generally recommended for pregnant women in cases of newly diagnosed SCH, such as with TSH levels greater than trimester-specific upper reference ranges, or greater than 4.0 mIU/L. Treatment may also be considered in pregnant women (or women trying to conceive) with TSH levels measuring 2.5 to 4.0 mIU/L and detectable TPO antibodies, but the available evidence is not consistent.20-22
Postpartum thyroiditis
Immune reconstitution after immune tolerance during pregnancy leads to an increased prevalence of autoimmune thyroid disorders in the first 12 months after the birth. Postpartum thyroiditis (PPT) is considered a variant form of HT. PPT occurs in 10% of unselected pregnant women and up to 50% of pregnant women with positive TPO antibodies, with recurrence rates approaching 70% with each subsequent pregnancy. Almost all cases occur after a full-term pregnancy; however, case reports have been described following a miscarriage.23-25
Although the classic triphasic pattern has been described in PPT, the pattern of thyroid dysfunction can be quite variable: 25 to 40% of patients exhibit the classic triphasic course, whereas 20 to 30% develop only thyrotoxicosis and 40% develop only hypothyroidism.25 The thyrotoxic phase may be asymptomatic, occurring up to one year after the birth and lasting for several weeks. This is followed by a hypothyroid phase, which is usually symptomatic. Diagnosis of PPT may be delayed as it is often under recognised and attributed to the stresses of having a newborn child. A prospective study reported that 50% of women with PPT remained hypothyroid at the end of the first postpartum year.24
There is likely to be an overlap between HT and PPT, given the autoimmune nature of both conditions. Monitoring TFT results two-monthly to evaluate for resolution of hypothyroidism for 12 months postpartum is recommended.
It is important to differentiate new-onset or recurrent Graves’ disease from the thyrotoxic phase of PPT. Features that are more suggestive of PPT include shorter duration of thyrotoxicosis, milder severity and the timing of the presentation within six months of delivery. Physical signs of thyroid ophthalmopathy, goitre with bruit and biochemical indicators of an elevated free tri-iodothyronine to free thyroxine ratio and presence of thyrotropin receptor antibodies or thyroid stimulating immunoglobulins are consistent with a diagnosis of Graves’ disease. Increased uptake on thyroid technetium scanning confirms a diagnosis of Graves’ disease; however, the scan is contraindicated during breastfeeding, which must be suspended until breast milk is no longer radioactive (usually several days). From a practical perspective, a thyroid technetium scan is typically not required, as improvement in repeat thyroid test results is seen within a few weeks. Thioamides are not used for management of PPT as they are in Graves’ disease.
Silent thyroiditis
Silent thyroiditis is considered a variant form of HT and is associated with the genes HLA-DR3 and HLA-DR5.26 The disease is more common in females and 50% of patients are positive for antibodies against thyroid peroxidase and thyroglobulin. Silent thyroiditis appears to be more prevalent in areas of higher dietary iodine intake, accounting for up to 30% of cases in Japan but around only 1% in the USA.27,28
The clinical course follows a triphasic pattern. The thyrotoxic phase is often transient and underdiagnosed, occurring in around 5 to 20% of patients, and is followed by a prolonged hypothyroid phase lasting three to six months.29 Symptoms are usually mild and a small painless goitre may be present. Residual chronic hypothyroidism occurs in 20% of patients.30 The main differential diagnosis to be considered for a presentation of thyrotoxicosis without neck pain is Graves’ disease.
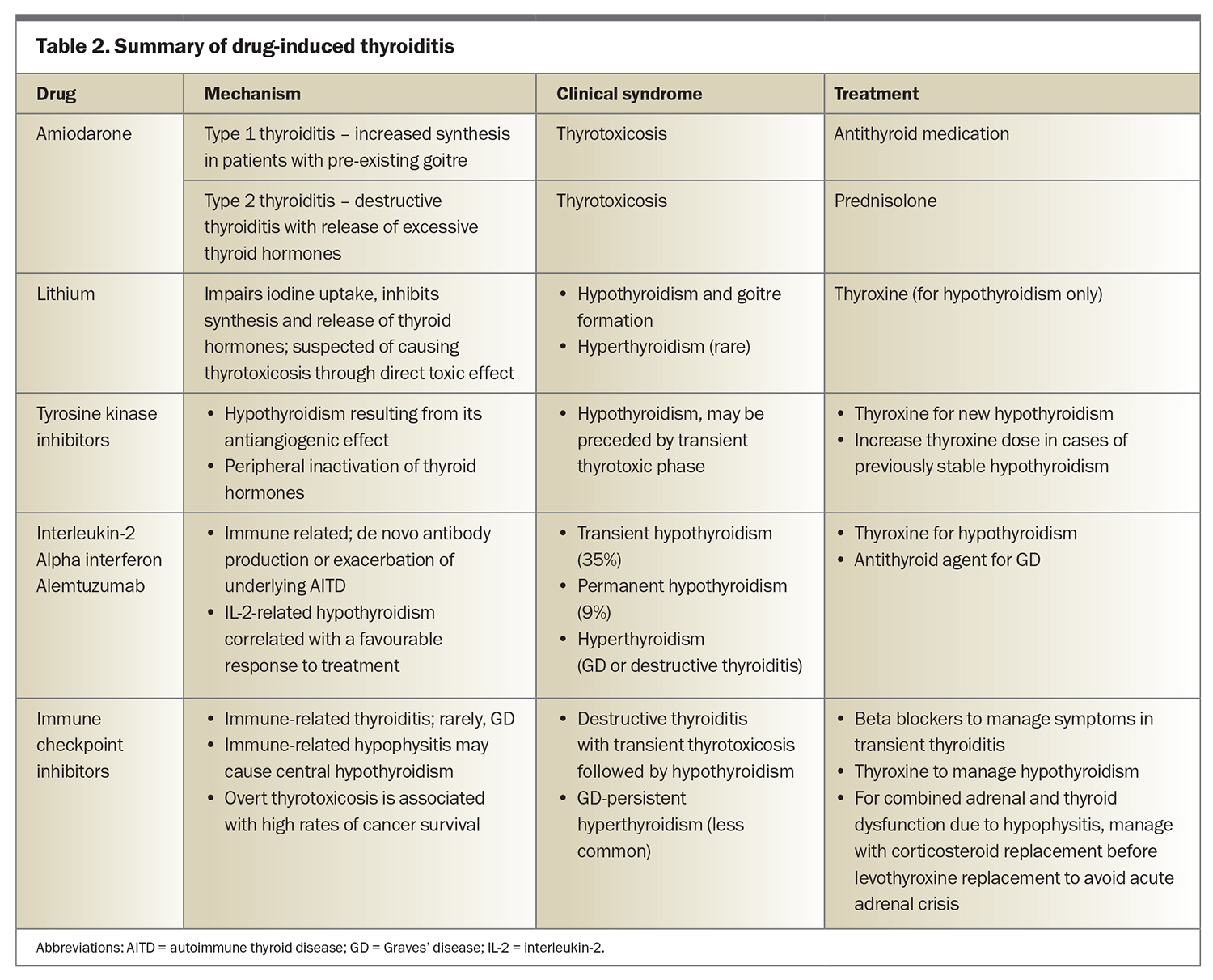
Drug-induced thyroiditis
Medications can cause thyroiditis through a variety of underlying mechanisms (Table 2). Altered iodine metabolism is seen with amiodarone-induced type 1 thyroiditis and causes increased thyroid hormone synthesis in patients with pre-existing goitre. This process results in thyrotoxicosis and responds well to management with antithyroid medications. Destructive thyroiditis with release of excessive thyroid hormones occurs with amiodarone-induced type 2 thyroiditis, which is best managed with prednisolone.
Lithium can impair iodine uptake and inhibit the synthesis and release of thyroid hormones, causing hypothyroidism and goitre formation in about 20% of affected patients.31
Tyrosine kinase inhibitors for cancer treatment can induce de novo hypothyroidism, which can be preceded by a phase of transient thyrotoxicosis in 20 to 40% of patients, resulting from the medication’s antiangiogenic effect.32 Worsening of stable hypothyroidism has also been described with the use of tyrosine kinase inhibitors.33
Medications that have a direct effect on the immune system exhibit an increased risk of autoimmune thyroid disease that presents most commonly as hypothyroidism, followed by destructive thyroiditis and Graves’ disease. Examples include IFN-alpha for viral hepatitis therapy, interleukin-2 therapy for cancer therapy and alemtuzumab (anti-CD52 monoclonal antibody) for multiple sclerosis.34-36
Immune checkpoint inhibitor therapies against cytotoxic T-lymphocyte-associated antigen 4 and programmed cell death protein 1 receptor are associated with thyroiditis and other endocrinopathies. Destructive thyroiditis is the most common immune-related endocrinopathy, occurring at a rate of 10 to 20% (especially with the use of combination therapy).33 Graves’ disease may also occur but is less common. Immune-related thyroiditis persists as hypothyroidism even after the completion of immune checkpoint inhibitor therapy. Immune-related hypophysitis may cause central hypothyroidism. In patients with combined adrenal and thyroid dysfunction due to hypophysitis, thyroid hormone replacement should begin three to five days after initiation of corticosteroid replacement to avoid an acute adrenal crisis. Overt thyrotoxicosis correlates with a robust immune response and high rates of extrathyroidal immune toxicity, as well as high rates of cancer survival.32
Subacute thyroiditis
Subacute thyroiditis is self-limiting and is the most common cause of painful thyroiditis. It is associated with viral infections or autoimmune responses and patients with certain human leucocyte antigens are more susceptible.37 A higher prevalence of subacute thyroiditis in women seems to correspond with the surge in viral infections that occurs with seasonal variability, including pathogens such as Coxsackie virus, Epstein-Barr virus, adenoviruses and H1N1 influenza.38 During the winter months of the pandemic, an increase in the incidence of subacute thyroiditis was observed in conjunction with spikes in cases of COVID-19.39 There are case reports of subacute thyroiditis following COVID-19 infection and, unusually, following COVID-19 vaccination.40
Subacute thyroiditis follows a triphasic pattern, with the initial thyrotoxic phase lasting up to six weeks marked by unilateral or bilateral anterior neck pain radiating to the jaw with associated neck tenderness on palpation. Prodromal features of fever, fatigue and myalgia associated with pharyngitis are usually present. Most patients return to euthyroid phase within 12 months of onset. Between 5 and 15% of patients develop permanent hypothyroidism and recurrence occurs in up to 5% of patients.29
Subacute thyroiditis can be diagnosed on the combination of neck pain with prodromal symptoms, thyrotoxicosis, elevated inflammatory markers and low uptake of markers on thyroid technetium scanning. Differential diagnoses include suppurative thyroiditis, Graves’ disease and haemorrhage into a thyroid cyst or nodule. Ultrasound is useful in delineating the features of a solid lesion (e.g. abscess, haemorrhage into nodule, thyroid cancer) that may require fine needle aspiration biopsy. For neck pain, NSAIDs are used as first-line therapy followed by the second-line option of corticosteroids, such as prednisolone 15 to 40 mg daily for several weeks followed by a gradual tapering of the dose. Beta blockers are used for symptomatic relief of thyrotoxicosis. Antithyroid medications are not effective in subacute thyroiditis.
Rare forms of thyroiditis
Acute suppurative thyroiditis or infectious thyroiditis
Acute thyroid infection leading to abscess is rare and can be a life-threatening condition if not recognised in a timely manner. Patients present with symptoms of neck pain. Predisposing mechanisms of infection include congenital anomalies, such as pyriform sinus fistula, direct extension of oropharyngeal abscess, lymphatic or haematogenous spread and neck trauma.41
The results of ultrasound-guided fine needle biopsy of the mass or collection of fluid help to differentiate between subacute thyroiditis and acute suppurative thyroiditis. Treatment of thyroid abscess includes hospitalisation, airway monitoring, stabilisation, empirical antibiotic therapy and drainage, if required.
Riedel thyroiditis
Riedel thyroiditis presents with a firm-to-hard goitre that is sometimes painful, with resulting compression causing symptoms of hoarseness, dyspnoea and dysphagia. It is a rare inflammatory disease related to immunoglobulin G-4, and adjacent tissues can be affected by invasive fibrosis thyroiditis.42 Airway management and corticosteroids are the mainstay of treatment alongside correction of biochemical abnormalities, such as hypocalcaemia and hypothyroidism.43
Radiation- and trauma-induced thyroiditis
Radiation-induced thyroiditis is uncommon and presents with anterior neck pain. It occurs within two weeks of external beam radiation or radio-iodine administration.44 Transient neck pain and thyrotoxicosis may occur after physical injury in situations including vigorous palpation, neck surgery, thyroid biopsy or seat belt injury.45
Conclusion
The underlying causes of thyroiditis are diverse, with presence or absence of neck pain and thyroid dysfunction being the main clinical presentations. The clinical context will determine which additional investigations are indicated to exclude important differential diagnoses. Depending on the underlying cause, treatment is generally aimed at managing the patient’s symptoms. Permanent hypothyroidism is a possible long-term complication of thyroiditis. Clinicians should be equipped with a working knowledge of thyroiditis and its various manifestations. ET
COMPETING INTERESTS: None.
References
1. Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab 2003; 88: 2100-2105.
2. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med 2003; 138: 315-318.
3. O’Leary PC, Feddema PH, Michelangeli VP, et al. Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clin Endocrinol (Oxf) 2006; 64: 97-104.
4. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995; 43: 55-68.
5. Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003; 88: 2983-2992.
6. Ferrari SM, Fallahi P, Ruffilli I, et al. The association of other autoimmune diseases in patients with Graves’ disease (with or without ophthalmopathy): review of the literature and report of a large series. Autoimmun Rev 2019; 18: 287-292.
7. Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med 1985; 312: 601-604.
8. Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 2010; 95: 496-502.
9. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014; 24: 1670-1751.
10. Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87: 3221-3226.
11. Rosario PW, Carvalho M, Calsolari MR. Natural history of subclinical hypothyroidism with TSH </=10 mIU/l: a prospective study. Clin Endocrinol (Oxf) 2016; 84: 878-881.
12. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376: 2534-2544.
13. Zhao M, Liu L, Wang F, et al. A worthy finding: decrease in total cholesterol and low-density lipoprotein cholesterol in treated mild subclinical hypothyroidism. Thyroid 2016; 26: 1019-1029.
14. Wang X, Wang H, Li Q, et al. Effect of levothyroxine supplementation on the cardiac morphology and function in patients with subclinical hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab 2022; 107: 2674-2683.
15. Monzani F, Caraccio N, Kozakowa M, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo- controlled study. J Clin Endocrinol Metab 2004; 89: 2099-2106.
16. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172: 811-817.
17. Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10: e0129793.
18. Sue LY, Leung AM. Levothyroxine for the treatment of subclinical hypothyroidism and cardiovascular disease. Front Endocrinol (Lausanne) 2020; 11: 591588.
19. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2: 215-228.
20. Nazarpour S, Ramezani Tehrani F, et al. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 2017; 176: 253-265.
21. Negro R, Schwartz A, Stagnaro-Green A. Impact of levothyroxine in miscarriage and preterm delivery rates in first trimester thyroid antibody-positive women with TSH less than 2.5 mIU/L. J Clin Endocrinol Metab 2016; 101: 3685-3690.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27: 315-389.
23. Stuckey BG, Kent GN, Ward LC, Brown SJ, Walsh JP. Postpartum thyroid dysfunction and the long-term risk of hypothyroidism: results from a 12-year follow-up study of women with and without postpartum thyroid dysfunction. Clin Endocrinol (Oxf) 2010; 73: 389-395.
24. Stagnaro-Green A, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Negro R. High rate of persistent hypothyroidism in a large-scale prospective study of postpartum thyroiditis in southern Italy. J Clin Endocrinol Metab 2011; 96: 652-657.
25. Lazarus JH. The continuing saga of postpartum thyroiditis. J Clin Endocrinol Metab 2011; 96: 614-616.
26. Farid NR, Hawe BS, Walfish PG. Increased frequency of HLA-DR3 and 5 in the syndromes of painless thyroiditis with transient thyrotoxicosis: evidence for an autoimmune aetiology. Clin Endocrinol (Oxf) 1983; 19: 699-704.
27. Nishimaki M, Isozaki O, Yoshihara A, Okubo Y, Takano K. Clinical characteristics of frequently recurring painless thyroiditis: contributions of higher thyroid hormone levels, younger onset, male gender, presence of thyroid autoantibody and absence of goiter to repeated recurrence. Endocr J 2009; 56: 391-397.
28. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003; 348: 2646-2655.
29. Samuels MH. Subacute, silent, and postpartum thyroiditis. Med Clin North Am 2012; 96: 223-233.
30. Nikolai TF, Coombs GJ, McKenzie AK. Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism and subacute thyroiditis. Long-term follow-up. Arch Intern Med 1981; 141: 1455-1458.
31. Bocchetta A, Bernardi F, Pedditzi M, et al. Thyroid abnormalities during lithium treatment. Acta Psychiatr Scand 1991; 83: 193-198.
32. Muir CA, Clifton-Bligh RJ, Long GV, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab 2021; 106: e3704-e3713.
33. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 173-182.
34. Deutsch M, Dourakis S, Manesis EK, et al. Thyroid abnormalities in chronic viral hepatitis and their relationship to interferon alfa therapy. Hepatology 1997; 26: 206-210.
35. Csaki AC, Blum M. Thyrotoxicosis after interferon-alpha therapy. Thyroid 2000; w10: 101.
36. Curti B, Daniels GA, McDermott DF, et al. Improved survival and tumor control with interleukin-2 is associated with the development of immune-related adverse events: data from the PROCLAIM(SM) registry. J Immunother Cancer 2017; 5: 102.
37. Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewinski A. Subacute thyroiditis is associated with HLA-B*18:01, -DRB1*01 and -C*04:01-the significance of the new molecular background. J Clin Med 2020; 9: 534.
38. Martino E, Buratti L, Bartalena L, et al. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest 1987; 10: 321-323.
39. Bostan H, Sencar ME, Calapkulu M, et al. Impact of the COVID-19 pandemic on the incidence, seasonal distribution, and characteristics of subacute thyroiditis. Endocrine 2023; 79: 323-330.
40. Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J Clin Endocrinol Metab 2020; 105: dgaa537.
41. Miyauchi A. Thyroid gland: a new management algorithm for acute suppurative thyroiditis? Nat Rev Endocrinol 2010; 6: 424-426.
42. Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 2012; 64: 3061-3067.
43. Falhammar H, Juhlin CC, Barner C, Catrina SB, Karefylakis C, Calissendorff J. Riedel’s thyroiditis: clinical presentation, treatment and outcomes. Endocrine 2018; 60: 185-192.
44. Mizokami T, Hamada K, Maruta T, Higashi K, Tajiri J. Painful radiation thyroiditis after (131)I therapy for Graves’ hyperthyroidism: clinical features and ultrasonographic findings in five cases. Eur Thyroid J 2016; 5: 201-206.
45. Dickey RA, Parker JL, Feld S. Discovery of unsuspected thyroid pathologic conditions after trauma to the anterior neck area attributable to a motor vehicle accident: relationship to use of the shoulder harness. Endocr Pract 2003; 9: 5-11.