Management of hyponatraemia: balancing salt and water

Hyponatraemia is a complex condition with varied clinical manifestations ranging from asymptomatic to severe and life-threatening. In an acute setting, severely symptomatic hyponatraemia requires urgent treatment, balanced with avoiding excessively rapid correction. Differential diagnosis, treatment of any underlying cause and maintaining an adequate sodium level to avoid symptoms are key in nonacute, asymptomatic cases.
- Hyponatraemia is the most common electrolyte disorder and is associated with long-term morbidity and mortality.
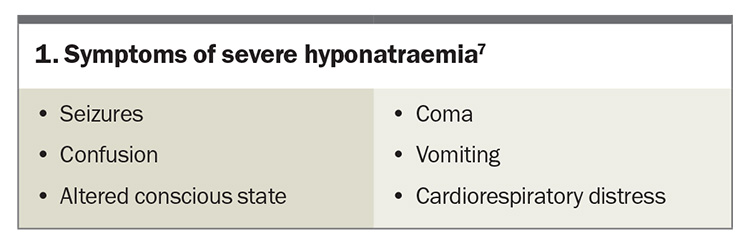
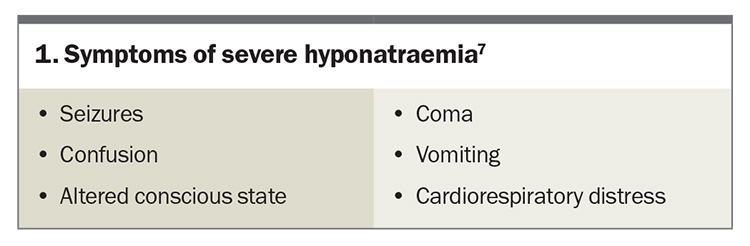
- Clinical manifestations of hyponatraemia vary significantly, from asymptomatic or nonspecific symptoms (e.g. vomiting) to life-threatening neurological complications (e.g. seizure, cerebral herniation).
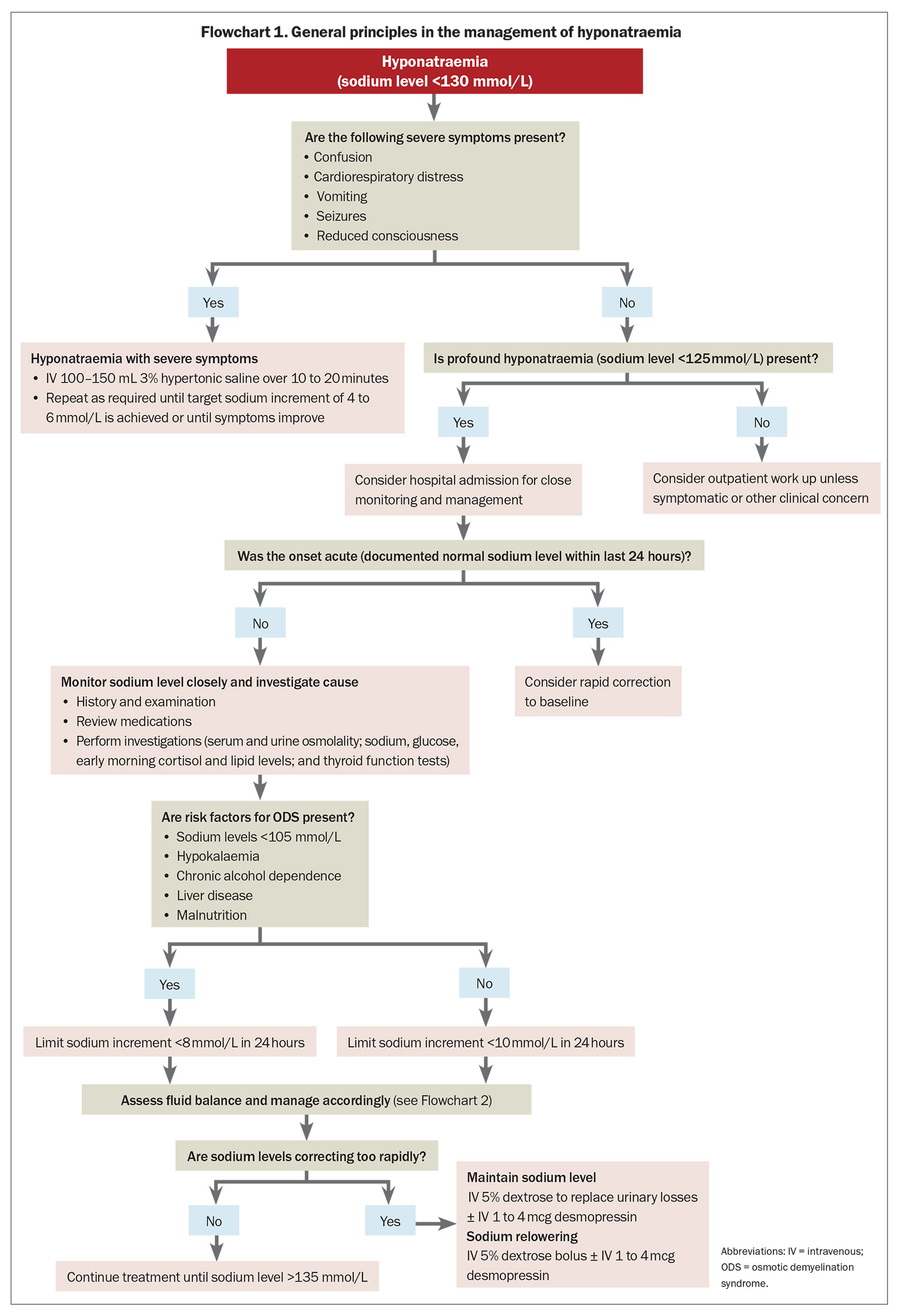
- Hyponatraemia with severe symptoms should be managed promptly with 3% hypertonic saline in hospital to reduce the risk of cerebral oedema.
- Increases in serum sodium levels should be limited to 10 mmol/L per day (or 8 mmol/L per day in people with risk factors for osmotic demyelination syndrome).
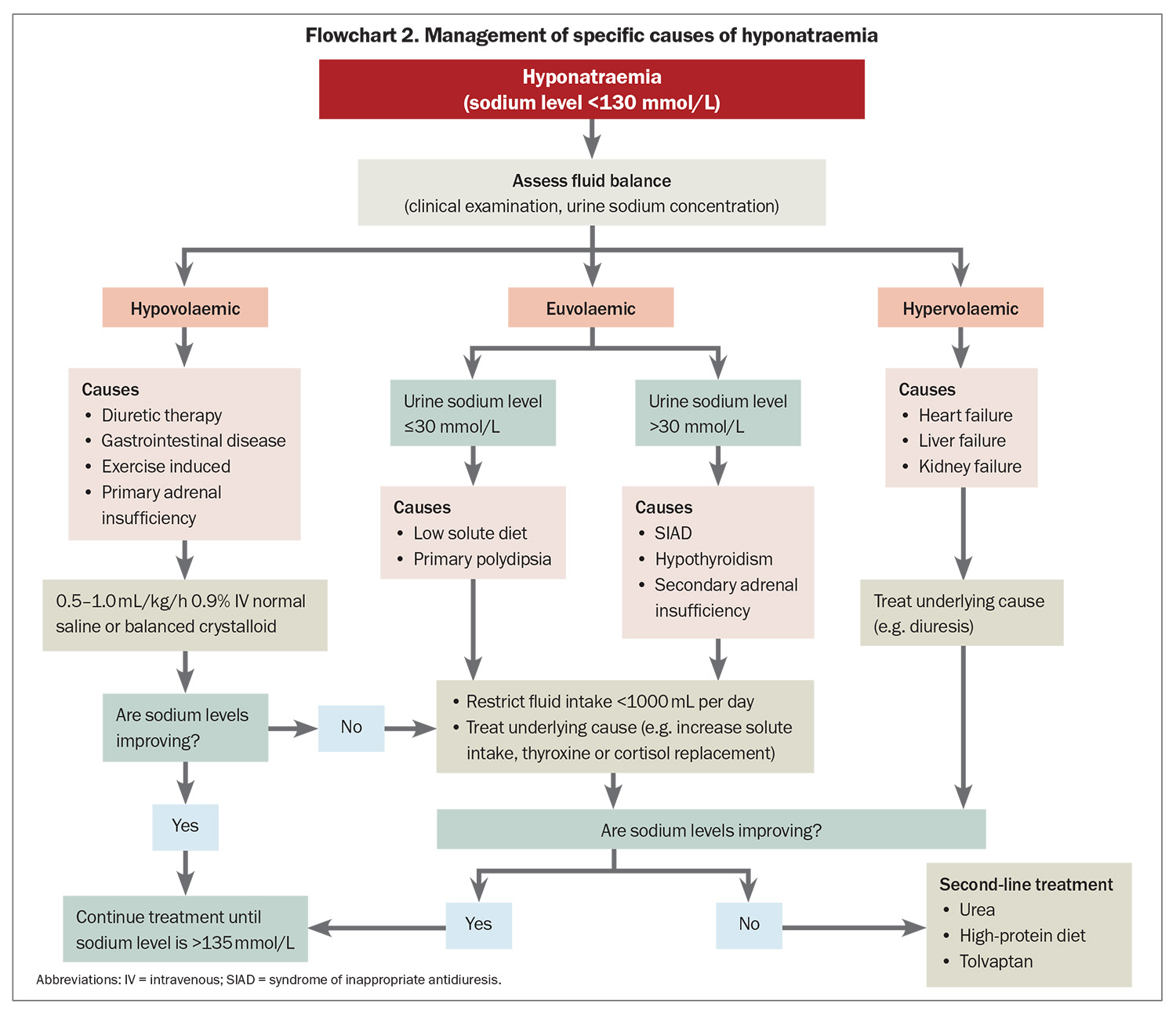
- The syndrome of inappropriate antidiuresis is the most common cause of hyponatraemia and first-line management is fluid restriction. Goals of treatment are reducing any symptoms and preventing hospital admissions. Second-line treatment options include urea powder, high-protein diet and tolvaptan.
Hyponatraemia is the most common electrolyte disorder, affecting 15 to 30% of hospitalised patients, and about 1 to 2% of the general population.1,2 It often results from water retention, which is disproportionate to total body sodium. Hyponatraemia is particularly prevalent in people with heart failure (30%), liver cirrhosis (50%) and people aged over 65 years (20%).3-5 Even mild hyponatraemia (defined below) is associated with increased hospital stay, morbidity and mortality, which translates to a high economic burden.1,6-8
Hyponatraemia is defined by a serum sodium level of less than 135 mmol/L. Biochemically, hyponatraemia is classified as mild (130 to 134 mmol/L), moderate (125 to 129 mmol/L) or profound (<125 mmol/L).7-9 The term severe hyponatraemia is reserved for those with severe symptoms such as seizures (see Box 1).7 Generally, acute hyponatraemia develops within 24 to 48 hours, whereas chronic hyponatraemia develops more gradually. Hyponatraemia can also be characterised by fluid status (hypovolaemic, euvolaemic or hypervolaemic), although clinical assessment of volume status is not always reliable.
Pathophysiology
Hyponatraemia is caused by a relative excess of free water compared with total body sodium content, often associated with vasopressin release. Arginine vasopressin (AVP, formerly known as antidiuretic hormone) mediates water balance. AVP release can be triggered by activation of osmoreceptors or baroreceptors, in response to hypovolaemia or hypotension.10 Among other effects, AVP triggers reabsorption of water in the kidneys and production of more concentrated urine. Sometimes AVP secretion can occur independently of usual triggers, or inappropriately, known as the syndrome of inappropriate antidiuresis (SIAD; formerly known as syndrome of inappropriate antidiuretic hormone secretion).
Low sodium levels result in an increased water shift across the blood–brain barrier into brain tissue, which can cause brain swelling. The brain tissue can adapt by reducing intracellular organic osmolytes, which takes about 48 hours.5,7,11 If hyponatraemia develops rapidly (e.g. water intoxication, exercise induced, MDMA induced), there is an increased risk of severe symptoms.5
Patients with chronic hyponatraemia whose brains have adapted to the low solute state are potentially at risk of osmotic demyelination syndrome (ODS) if the serum sodium level rises too rapidly.12 ODS is a rare but devastating neurological complication and risk factors include a serum sodium level of less than 105 mmol/L, hypokalaemia, chronic alcohol dependence, liver disease and malnutrition.7,11,12 ODS may have a delayed presentation of up to one week following overcorrection of hyponatraemia, with symptoms such as hyperreflexia, pseudobulbar palsy, parkinsonism, quadriparesis and death.12 Hence, management of severe hyponatraemia requires a careful balance to avoid the complications of both very low, or rapidly rising, sodium levels.
Presentation
The clinical manifestations of hyponatraemia can vary significantly from asymptomatic to severe and life-threatening.7,11 Hyponatraemia can present with vague symptoms such as weakness, nausea or headache. Even patients with chronic hyponatraemia who appear asymptomatic can have gait disturbances, falls, fractures, impaired concentration and cognitive deficits.13
Severe symptoms of hyponatraemia are caused by cerebral oedema and are more common if the sodium level has dropped acutely. Severe symptoms of hyponatraemia are listed in Box 1.7 Patients with a newly identified sodium level of less than 125 mmol/L or associated symptoms will often require hospital admission.
Common causes of hyponatraemia
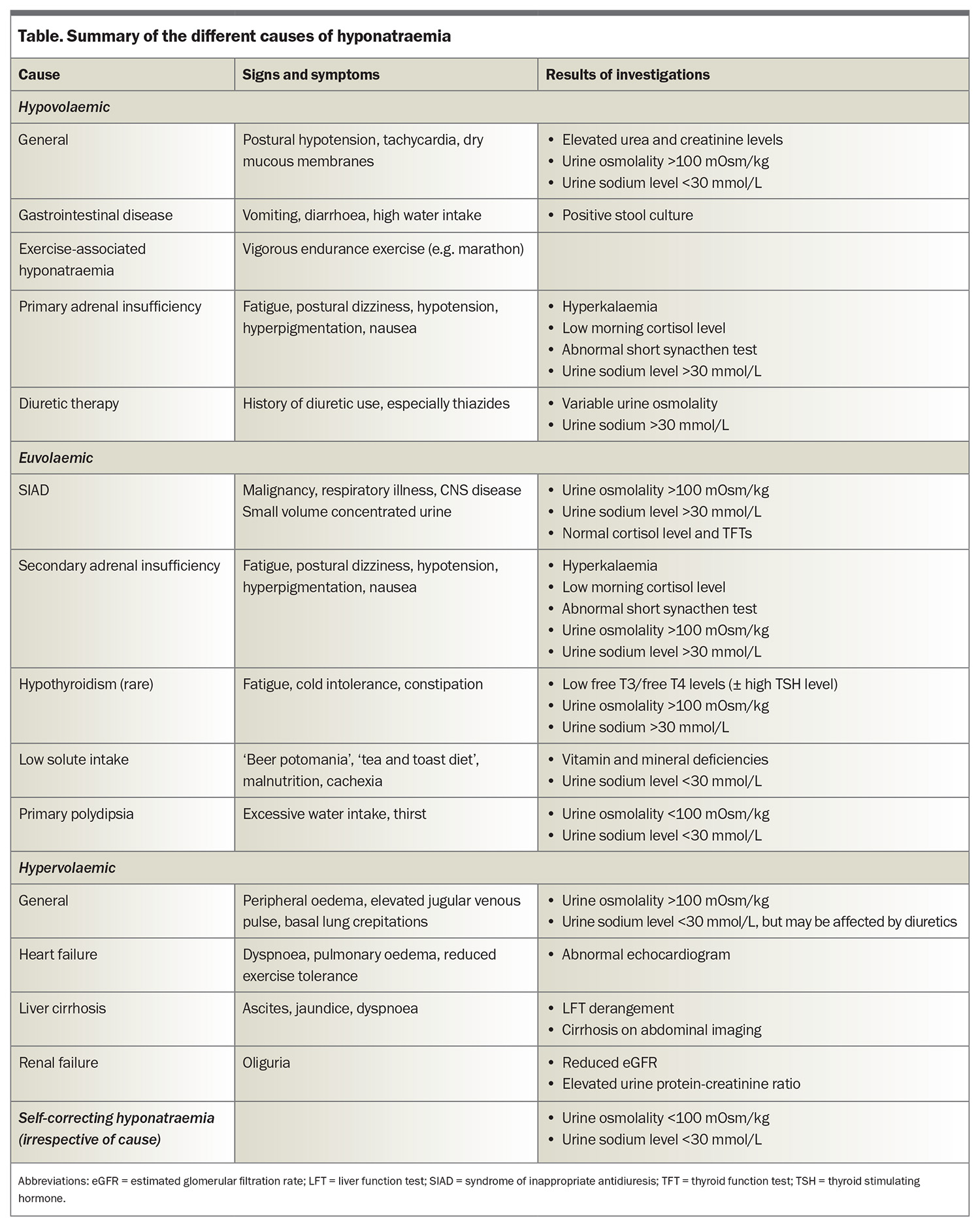
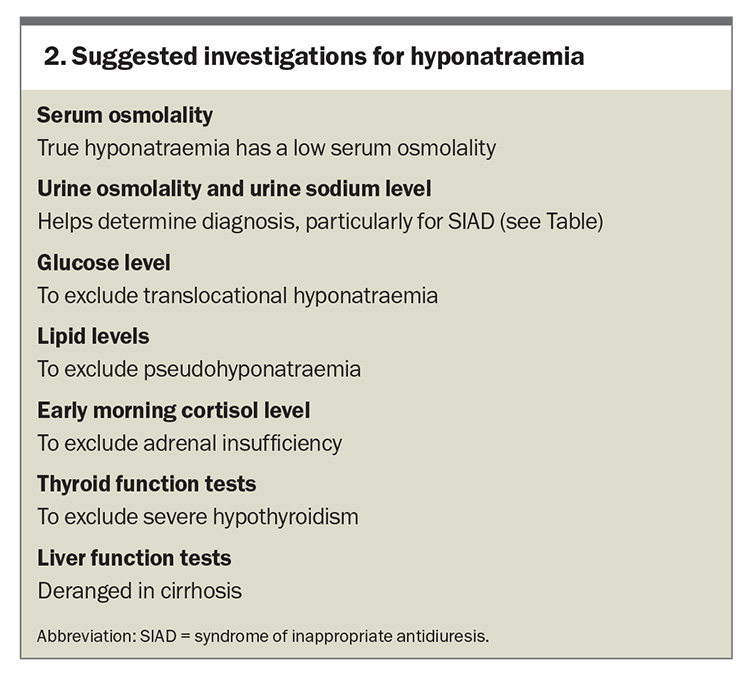
It is important to establish whether hyponatraemia is truly present (hypotonic hyponatraemia) or whether there are other factors affecting the laboratory result. If lipid or protein levels are markedly elevated, then the sodium level measurement via urea, electrolytes and creatinine can be inaccurate. The sodium level should, therefore, be measured directly using a blood gas analyser.7 The presence of additional solutes (e.g. glucose, mannitol, radiographic contrast) can cause a low serum sodium level by drawing water into the circulation. These cases do not require any specific treatment, and can be differentiated from true hyponatraemia by a high or normal serum osmolality. True hyponatraemia is associated with a low serum osmolality. A summary of the causes of hyponatraemia is provided in the Table and suggested investigations are listed in Box 2.
Hypovolaemic hyponatraemia is driven by appropriate AVP secretion in response to reduced circulating volume, resulting in diminished electrolyte–free water clearance.7 Sodium losses can occur via the gastrointestinal tract (e.g. diarrhoea), transdermally (e.g. sweating, burns) or renally (e.g. diuretics, primary adrenal insufficiency, renal disease). Thiazide diuretics are a common cause of hyponatraemia, and can be associated with a genetic predisposition, therefore, patients who develop thiazide-associated hyponatraemia should avoid them in the future to prevent recurrence. Hypovolaemic hyponatraemia is treated by volume replacement with 0.9% (normal) saline and addressing the underlying cause.
Hypervolaemic hyponatraemia is associated with reduced effective arterial blood volume and baroreceptor-mediated AVP release. Hypervolaemic hyponatraemia may be caused by pump failure (heart failure), reduced vascular resistance (liver failure) or arterial underfilling (renal failure with proteinuria).5 The best approach is treatment of the underlying cause, potentially with fluid restriction, loop diuretics and other disease-specific treatment as appropriate.
SIAD is the most common cause of hyponatraemia. SIAD can be caused by malignancies, CNS disorders, lung disorders, medications, stress (e.g. pain and nausea) or genetic defects, or it may be idiopathic (Box 3).14 SIAD is a diagnosis of exclusion. Adrenal insufficiency is an important differential diagnosis that can mimic SIAD,so it is imperative to measure an early morning serum cortisol level.15 Rarely, extreme hypothyroidism may also resemble SIAD.
Excessive water intake and low solute intake, either in the short or long term, can also cause hyponatraemia, reflected by a low urine osmolality (usually <100 mOsm/kg) and low urine sodium level (usually <20 to 30 mmol/L).7 The mainstay of treatment is fluid restriction, as well as increasing dietary solute intake. Ice chips and sour candies can be effective in alleviating mouth dryness in primary polydipsia.7,11
Management of hyponatraemia with severe symptoms
Hyponatraemia with severe symptoms must be treated urgently to prevent severe neurological consequences.5,16 Studies have shown that a 4 to 6 mmol/L increase in serum sodium concentration is sufficient to improve severe symptoms of hyponatraemia, reducing intracranial pressure by 50%.17 A bolus of 3% hypertonic saline is an effective way to increase the serum sodium concentration by this modest interval.7 Guidelines recommend that hyponatraemia associated with severe symptoms, regardless of chronicity, should be managed with intravenous 100 to 150 mL 3% hypertonic saline over 10 to 20 minutes. If necessary, this can be repeated until the target sodium increment of 4 to 6 mmol/L is achieved or until the symptoms improve, whichever comes first (see Flowchart 1).7,11 Patients with severe hyponatraemia are typically managed in an intensive care or high dependency setting.7 Sodium levels should be monitored closely to ensure correction limits are not exceeded.
Rate of correction
Guidelines recommend that the rise in serum sodium levels should be limited to 10 mmol/L in the first 24 hours, and 18 mmol/L in the first 48 hours to minimise the risk of ODS.7,11 For patients at high risk of ODS, sodium increments should be restricted further to less than 8 mmol/L in any 24-hour period.11 In at-risk patients, the sodium concentration must be monitored closely at least every four to eight hours to detect overcorrection. Sudden polyuria (>100 mL/hour) can be a sign of impending overcorrection, and in these cases, sodium concentration should be monitored every one to two hourly.7
Preventing overcorrection
If the rise in serum sodium concentration exceeds targets, any ongoing hyponatraemia treatment should be discontinued.7,11 Serum sodium concentration can be held steady by replacing urinary losses with 5% dextrose or by halting urine output with 1 to 4 mcg of intravenous desmopressin. Alternatively, or in combination, relowering of sodium concentration can be achieved by a bolus of 5% dextrose or a combination of 5% dextrose and desmopressin to achieve a net sodium rise within the acceptable 24-hour limit.7,11
Management of chronic hyponatraemia without severe symptoms
The main goal of chronic hyponatraemia management is to maintain an adequate sodium level to prevent hyponatraemia symptoms and hospital admission (see Flowchart 2). The same recommended rates of correction outlined above apply for patients without severe symptoms because they may be at risk of ODS if their serum sodium level is below 125 mmol/L. There is no clear evidence for specific long-term treatment targets for chronic hyponatraemia. Aiming for a serum sodium level of more than 130 mmol/L is a common target. In patients who are stable and asymptomatic, a target level of more than 125 mmol/L may be appropriate.
First-line treatment
The first-line treatment of SIAD, the most common cause of hyponatraemia especially in older people, is fluid restriction.7,11 The effectiveness of restriction will depend on urine output. An observational study involving 106 outpatients with SIAD found that 59% responded to 1 L fluid restriction, with predictors of nonresponse being a more concentrated urine at baseline (urine sodium level ≥130 mmol/L and urine osmolality ≥500 mOsm/kg).18
Second-line treatments
Second-line treatments of SIAD include increasing dietary solute intake (protein, urea) and use of AVP receptor antagonists.7,11 Urea, a product of nitrogen metabolism that can be administered orally to increase water excretion in urine, has been shown to be effective for people with SIAD in observational studies.19 The typical dose is 15 to 60 g daily, but often at least 30 g per day is required for efficacy.20 The main limitation of urea is bitter taste, which can be improved by using a commercial flavoured preparation or dissolving plain urea powder in fruit juice. Urea is produced in the body via protein metabolism, so it has also been explored whether increasing dietary protein intake could also improve sodium levels. A recent pilot study comparing urea 30 g daily with a 90 g protein shake supplement found similar improvements in serum sodium concentration.21
AVP V2-receptor antagonists, such as tolvaptan, inhibit the action of AVP in the renal collecting ducts, resulting in water diuresis.22 Tolvaptan has been shown to be very effective at raising sodium levels in large randomised controlled trials.23 The typical dose is 7.5 to 15 mg daily as a single dose, with ongoing therapy reassessed daily. However, tolvaptan has several limitations, including risk of rapid sodium rise (overcorrection), high cost and potential liver injury if used in higher doses or for a long duration. It is typically prescribed in hospital to facilitate close monitoring (sodium level monitoring initially every six hours), and patients should be allowed to drink freely to reduce the risk of overcorrection.11 Tolvaptan is used infrequently as a long-term agent, but can be effective if other strategies are not successful.
There is limited evidence for the use of salt tablets in the treatment of hyponatraemia. Some studies have shown a small but significant improvement in sodium levels with the use of salt tablets, whereas other studies have shown no benefit.24,25 Moreover, more than 10 salt tablets per day are required to have a similar osmotic effect as 15 g of urea. The intense salty taste of salt tablets often leads to excessive fluid consumption, which can be counterproductive. Frusemide and demeclocycline are not effective in the treatment of hyponatraemia, and can increase the risk of kidney injury.
Conclusion
Hyponatraemia is the most common electrolyte disorder. It is a complex condition that is associated with long-term morbidity and mortality. In patients with severe symptoms, prompt administration of hypertonic saline is critical. Treatment of mild to moderate chronic hyponatraemia is directed at the underlying disease process, according to volume status and serum/urine biochemistry. For patients with chronic hyponatraemia caused by SIAD, the first-line treatment is fluid restriction. Second-line treatments include urea powder, high protein diet and tolvaptan. ET
COMPETING INTERESTS: Dr Lin: None. Dr Warren has received research funding for an investigator-initiated trial from Otsuka Australia Pharmaceutical.
References
1. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med 2006; 119(7 Suppl 1): S30-S35.
2. Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126: 1127-1137.e1.
3. Davila CD, Udelson JE. Hypervolemic hyponatremia in heart failure. Front Horm Res 2019; 52: 113-129.
4. Solà E, Ginès P. Hypervolemic hyponatremia (Liver). Front Horm Res 2019; 52: 104-112.
5. Adrogué HJ, Tucker BM, Madias NE. Diagnosis and management of hyponatremia: a review. JAMA 2022; 328: 280-291.
6. Verbalis J. Hyponatremia and hypoosmolar disorders. 2018. p. 68-76.e1.
7. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014; 170: G1-47.
8. Boscoe A, Paramore C, Verbalis JG. Cost of illness of hyponatremia in the United States. Cost Eff Resour Alloc 2006; 4: 10.
9. Spasovski G. Hyponatraemia — treatment standard 2024. Nephrol Dial Transplant 2024; 39: 1583-1592.
10. Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 2003; 17: 471-503.
11. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013; 126(10 Suppl 1): S1-42.
12. Sterns RH. Adverse consequences of overly-rapid correction of hyponatremia. Front Horm Res 2019; 52: 130-142.
13. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119(1): 71.e1-8.
14. Feldman BJ, Rosenthal SM, Vargas GA, et al. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 2005; 352: 1884-1890.
15. Faustini-Fustini M, Anagni M. Beyond semantics: defining hyponatremia in secondary adrenal insufficiency. J Endocrinol Invest 2006; 29: 267-269.
16. Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med 1986; 314: 1529-1535.
17. Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol 2009; 29: 282-299.
18. Winzeler B, Lengsfeld S, Nigro N, et al. Predictors of nonresponse to fluid restriction in hyponatraemia due to the syndrome of inappropriate antidiuresis. J Intern Med 2016; 280: 609-617.
19. Wendt R, Fenves AZ, Geisler BP. Use of urea for the syndrome of inappropriate secretion of antidiuretic hormone: a systematic review. JAMA Netw Open 2023; 6(10): e2340313.
20. Lockett J, Berkman KE, Dimeski G, Russell AW, Inder WJ. Urea treatment in fluid restriction-refractory hyponatraemia. Clin Endocrinol 2019; 90: 630-636.
21. Monnerat S, Atila C, Baur F, et al. Effect of protein supplementation on plasma sodium levels in the syndrome of inappropriate antidiuresis: a monocentric, open-label, proof-of-concept study-the TREASURE study. Eur J Endocrinol 2023; 189: 252-261.
22. Decaux G, Gankam Kengne F. Hypertonic saline, isotonic saline, water restriction, long loops diuretics, urea or vaptans to treat hyponatremia. Expert Rev Endocrinol Metab 2020; 15: 195-214.
23. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006; 355: 2099-2112.
24. Spanuchart I, Watanabe H, Aldan T, Chow D, Ng RCK. Are salt tablets effective in the treatment of euvolemic hyponatremia? South Med J 2020; 113: 125-129.
25. Krisanapan P, Vongsanim S, Pin-On P, Ruengorn C, Noppakun K. Efficacy of furosemide, oral sodium chloride, and fluid restriction for treatment of syndrome of inappropriate antidiuresis (SIAD): an open-label randomized controlled study (The EFFUSE-FLUID Trial). Am J Kidney Dis 2020; 76: 203-212.