Differentiated thyroid cancer: an individualised management approach
The incidence of thyroid nodules and thyroid cancer is increasing. Most differentiated thyroid cancer cases have an indolent course with favourable survival outcomes. Management should be individualised and involve a dynamic and multidisciplinary team approach, with consideration of patient preference to avoid over treatment of low-risk disease and under treatment of high-risk disease.
- The incidence of thyroid cancer is increasing, with most cases being incidentally diagnosed on ultrasound.
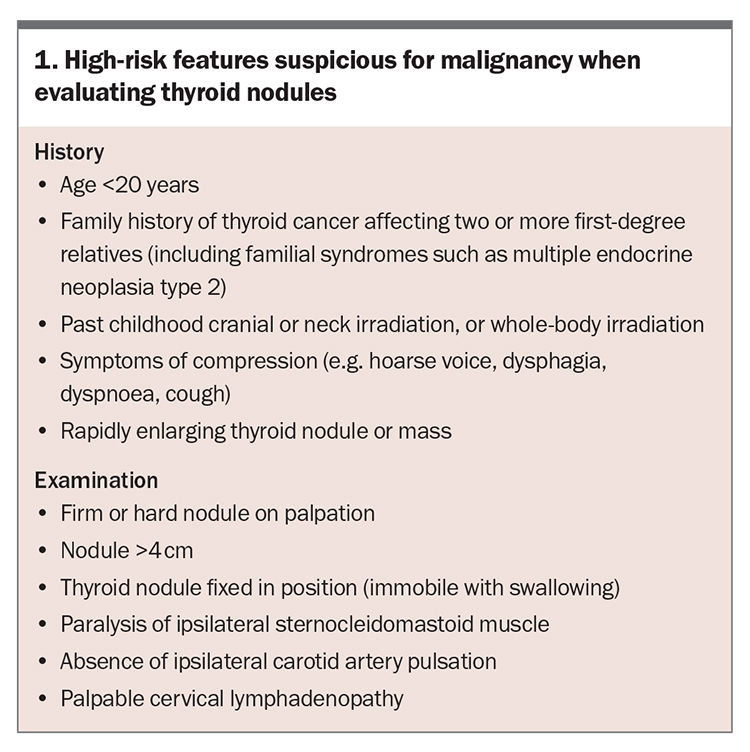
- Thyroid nodules should be carefully evaluated for suspicious clinical and sonographic features (including age, family history and the appearance, size and rate of growth of the nodule) to determine if further investigation with fine-needle aspirate biospy is required.
- Thyroglobulin (Tg) assessment should only occur post-thyroidectomy, as there is no appropriate reference range for its interpretation before surgery.
- Management is individualised and, for advanced disease, may be guided by a multidisciplinary team. The goal is to stratify risk early and recommend appropriate next steps (extent of surgery, radioactive iodine dosing and suitability for use of tyrosine kinase inhibitors).
- Serial clinical evaluation of thyroid-stimulating hormone, Tg and thyroglobulin antibody levels is required to assess disease response post-thyroidectomy. Serial Tg and thyroglobulin antibody measurements must be performed together to allow for appropriate interpretation.
- Dynamic risk stratification should be performed at each subsequent visit for continuous assessment of disease risk, based on treatment response.
- Tyrosine kinase inhibitors have been shown to delay disease progression in patients with radioiodine-refractory thyroid cancer. Currently, lenvatinib is the only tyrosine kinase inhibitor that is PBS subsidised for this indication.
The incidence of both thyroid nodules and thyroid cancer is increasing worldwide.1-4 In 2018, thyroid cancer was the ninth most common cancer diagnosed in Australia and accounted for 2.5% of all new cancer diagnoses. One in 80 (1.3%) people in Australia by the age of 85 years will be diagnosed with thyroid cancer. The increase in incidence is mostly because of the increased use of medical imaging and detection of early-stage papillary thyroid cancers (PTC). Despite this, thyroid cancer mortality is low and accounts for 0.3% of all cancer-related deaths and carries a favourable five-year survival of 97%, compared with 70% across all cancer types.5,6
There are four main types of thyroid cancer: PTC (arising from follicular cells), follicular thyroid cancer (FTC, also arising from follicular cells), anaplastic thyroid cancer (a more aggressive form of follicular cell-derived thyroid cancer) and medullary thyroid cancer (arising from parafollicular C-cells). Differentiated thyroid cancer (DTC) includes: PTC, which accounts for about 90% of all thyroid cancers; FTC, which accounts for about 5% of all thyroid cancers; and a rare subtype HÜrthle cell cancer. This article focuses on the diagnosis and management of DTC.
Presentation
Thyroid nodules are common in the general population and become more frequent with older age. Reassuringly, most thyroid nodules are benign. The most common presentation of thyroid cancer is through an incidental finding of thyroid nodules on imaging or with a lump in the neck. Appropriate risk assessment of thyroid nodules is required to facilitate correct identification of suspicious nodules, which warrant ongoing surveillance and diagnostic procedures.7
Clinical assessment
Assessment of thyroid nodules should begin with a clinical evaluation, including thorough history taking and an examination to identify suspicious features for malignancy. Risk factors for malignancy on history taking include a positive family history of thyroid cancer and past cranial, neck or whole-body irradiation. Red-flag symptoms suspicious for malignant thyroid nodules include rapidly enlarging nodules or symptoms of compression, such as voice hoarseness or dysphagia. On examination, benign nodules are usually soft and mobile with swallowing, whereas malignant thyroid nodules may be fixed in position, invading adjacent structures and associated with palpable cervical lymphadenopathy.
Diagnostic workup
The diagnostic workup of patients with a palpable thyroid nodule should begin with a serum thyroid-stimulating hormone (TSH) measurement and neck ultrasound, followed by a fine-needle aspirate biopsy (FNAB) if indicated. Malignant nodules are usually nontoxic and, therefore, present with a TSH level that is either elevated or within the normal range. It is important to include relevant history-taking and examination findings on the radiology request form for neck ultrasound, particularly if there are any clinical features suspicious for malignancy (Box 1). If concerned about malignancy, ensure to request sonographic examination of the cervical lymph nodes as well. There is no role in performing a preoperative assessment of thyroglobulin (Tg) levels, as there is no appropriate reference range for its interpretation. Tg levels can be elevated in benign and malignant disease. As such, Tg assessment should be confined to the post-thyroidectomy period to enable risk assessment for DTC.
Nodule risk assessment and the role of FNAB
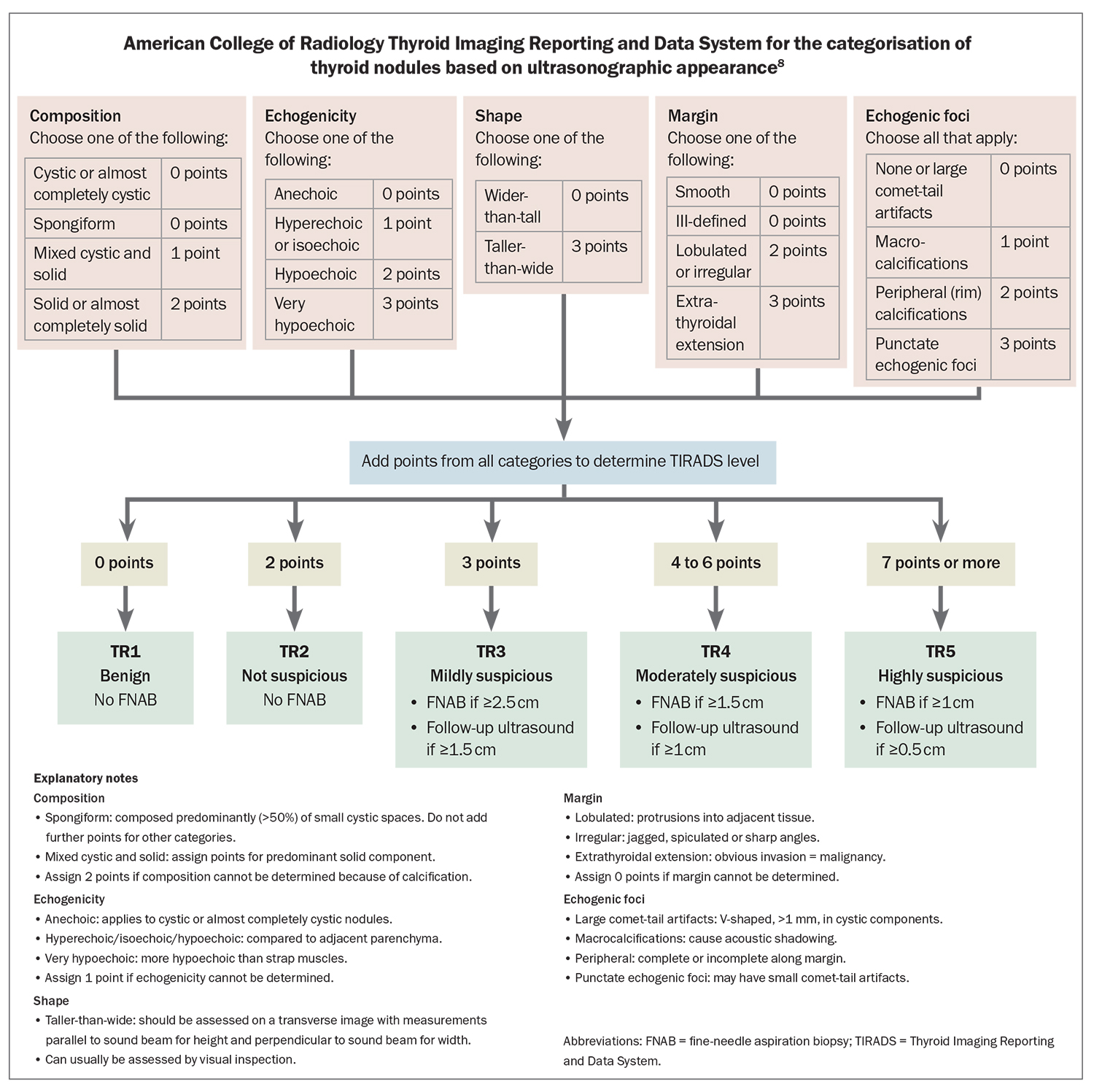
Radiological scoring systems provide a lexicon to describe thyroid nodules seen on ultrasound and provide a standardised approach to guide further investigation and management according to the presence of suspicious features and likelihood of malignancy. These scoring systems include the American College of Radiology Thyroid Imaging Reporting and Data System (ACR-TIRADS) (Flowchart) and the American Thyroid Association (ATA) 2015 guidelines (recommendation 8), both of which are helpful when included in the sonography report.8,9
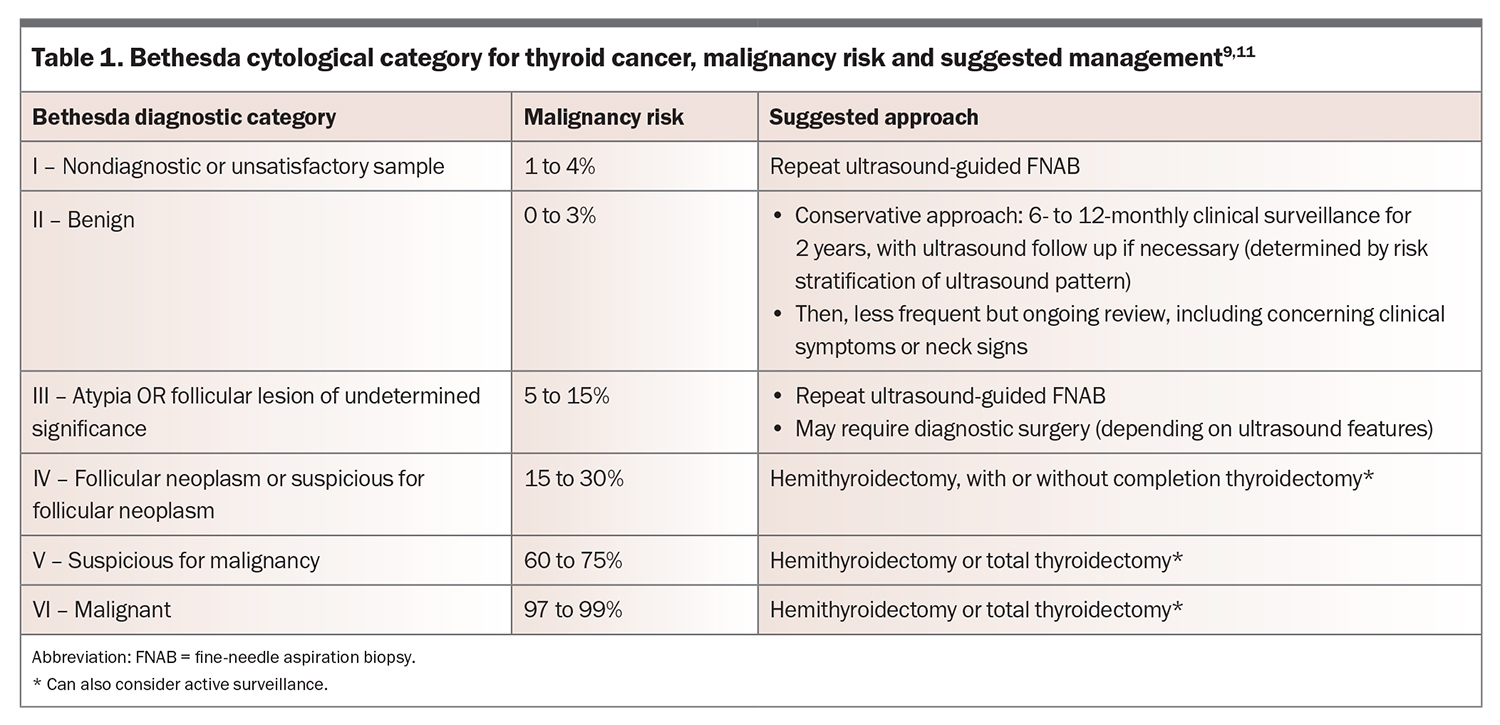
The approximate risk of malignancy within a nodule rises with an increasing TIRADS score and is estimated at: 2% or less for TIRADS 1 and 2; 5% or less for TIRADS 3; 20% or less for TIRADS 4 nodule; and more than 20% for TIRADS 5 nodules.8 Such information can be helpful when discussing the rationale of proceeding to an ultrasound-guided FNAB, which is indicated for any thyroid nodule measuring 1 cm or greater with suspicious sonographic features (recommendation 8, ATA 2015 guidelines).9 Importantly, these scoring systems are a guide only, and should be used as a screening tool to determine the nodules that require further investigation with FNAB. If unsure of the likelihood of malignancy of a nodule, there should be a low threshold for referring the patient to an endocrinologist. Aspirated material is sent to a laboratory for cytological assessment and classified according to the Bethesda classification system, which provides a correlative risk of malignancy and suggested management.
Bethesda classification of thyroid nodules
The Bethesda System for Reporting Thyroid Cytopathology was published in 2007 and classifies the cytology of thyroid nodules into one of six categories that correlate with the risk of malignancy (Table 1).11 Importantly, most thyroid nodules are benign. Bethesda IV nodules have a greater likelihood of malignancy if subcategorised as follicular neoplasm rather than suspicious for follicular neoplasm; however, it is impossible to distinguish follicular adenoma from carcinoma on FNAB, as the defining features of capsular and vascular invasion cannot be evaluated cytologically. Therefore, surgery for Bethesda IV nodules is largely for the purpose of diagnosis.
Thyroid surgery: the hallmark of initial thyroid cancer management
Most DTC is diagnosed at the stage of small, indolent disease, before the development of nodal or distant metastases. Fewer than 10% of patients with DTC develop distant metastases, with the most common sites being the lungs and bone.12
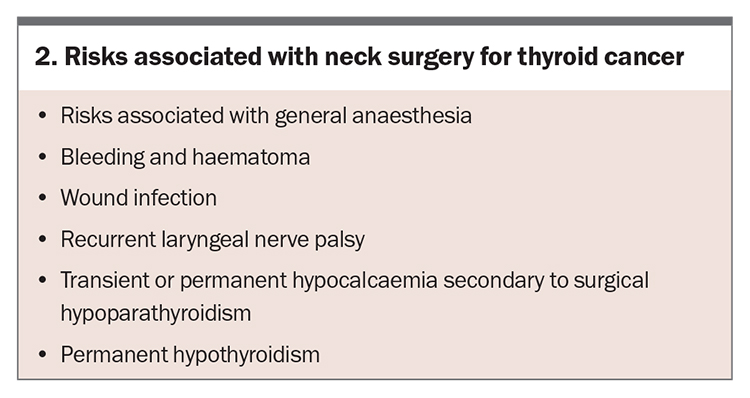
A personalised management approach is preferred, with options including active surveillance for very low-risk disease or definitive neck surgery. Most thyroid cancers can be successfully treated with surgery. The primary goal of definitive neck surgery is removal of the primary tumour, locally invasive disease (i.e. disease that has extended beyond the thyroid capsule) and any lymph node metastases.9 Total thyroidectomy can result in lower recurrence rates and improved survival; however, it can be complicated by recurrent laryngeal nerve injury, hypoparathyroidism and lifelong hypothyroidism.13 Therefore, an individualised decision on the extent of surgery should be made based on disease burden, patient characteristics and risks of thyroid surgery. Hemithyroidectomy may be suitable in some cases of low-risk disease (e.g. ≤4 cm tumour confined to the thyroid with absence of gross extrathyroidal extension [ETE] and absence of nodal/distant metastases on ultrasound or at surgery), otherwise the standard of care is total thyroidectomy, particularly if there is also a family history of DTC.13,14 Neck dissection is also performed if there is evidence of local invasion or lymph node involvement.9
Perioperative risks with thyroid surgery include recurrent laryngeal nerve palsy and postoperative hypoparathyroidism, which is most commonly transient, but may be permanent (Box 2). Institution-specific rates of perioperative risks, if available, should ideally be discussed with the patient by the thyroid surgical team. Levothyroxine is required lifelong for hypothyroidism post-total thyroidectomy and may also be recommended for TSH suppression in intermediate- or high-risk cases (Table 2). Rates of hypothyroidism needing thyroxine supplementation after hemithyroidectomy are varied across studies and can range from 20% at three years to more than 50% at five years,15,16 but there is limited evidence to guide TSH suppression in these patients.17 In complex cases, such as locally invasive disease or lymph node metastases, referral to a high-volume thyroid surgeon, who should ideally provide surgeon- or centre-specific complication rates, is preferred.
What’s new: active surveillance
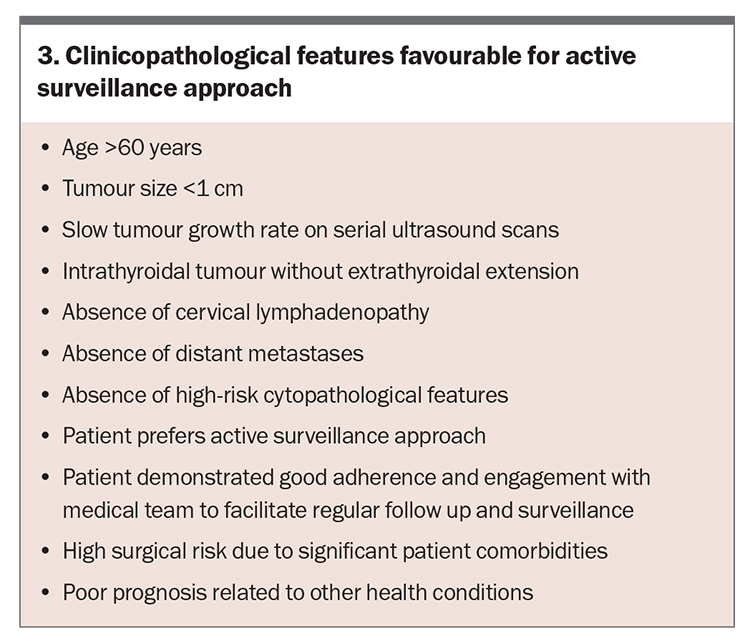
Over the past decade, active surveillance (i.e. surveillance without surgery) has emerged as a management option in patients with very low-risk disease (e.g. papillary thyroid microcarcinoma <1 cm, without ETE or nodal or distant metastases). In such cases, active surveillance has been shown to be safe and effective over five to 10 years of follow up, primarily because of the slow rate of growth and low risk of disease progression in these small indolent tumours.14,18 Clinicopathological features considered favourable for an active surveillance approach are summarised in Box 3. Active surveillance should occur in a multidisciplinary team setting, involving an endocrinologist or endocrine surgeon.
Initial postoperative risk stratification
The management of DTC has shifted from a ‘one size fits all’ approach to tailored therapeutic decision-making that considers patient preference, disease-specific factors and treatment response. Postoperatively, patients should undergo initial risk stratification to evaluate the need for further treatment, particularly with radioactive iodine (RAI), and to guide the aggressiveness of TSH suppression with levothyroxine. The purpose of risk stratification is to categorise patients in terms of their risk of disease recurrence as opposed to mortality, which remains low in this cohort. Risk stratification should occur through both:
- the American Joint Committee on Cancer (AJCC) tumour, node, metastasis (TNM) 8th edition staging system, which is used for initial staging, to predict the overall and disease-specific survival and need for adjuvant RAI;19 and
- the ATA risk stratification system, which is used to predict the risk of disease recurrence and to guide treatment decisions at each follow-up visit.9
In 2018, the AJCC/TNM 8th edition staging system raised the ‘age of diagnosis’ criteria from 45 to 55 years of age, and limited patients aged 55 years or above with lymph node metastases to being categorised as having stage II disease.20 This change in criteria saw the down-classification of many patients to stage I disease and highlighted the importance of using the ATA system to predict the risk of disease recurrence.
The ATA 2015 guidelines stratify patients into low, intermediate or high risk, based on the intraoperative and histopathological features from the tumour specimen.9 Features in the ATA risk stratification guidelines include the size of the tumour, multifocality, the presence of nodal disease, capsular invasion, lymphovascular invasion, ETE, if the surgical margins are involved with the tumour and postoperative serum Tg and TgAb levels. The risk of persistent structural or recurrent disease in low-, intermediate- and high-risk groups one year after initial stratification is about 3%, 18% and 66%, respectively.20 Serum-specific molecular profiling (e.g. BRAF V600E, TERT promotor mutations) by somatic mutation testing on tumour specimens can also be incorporated to predict the risk of ETE, lymph node and distant metastases.
Postoperative management
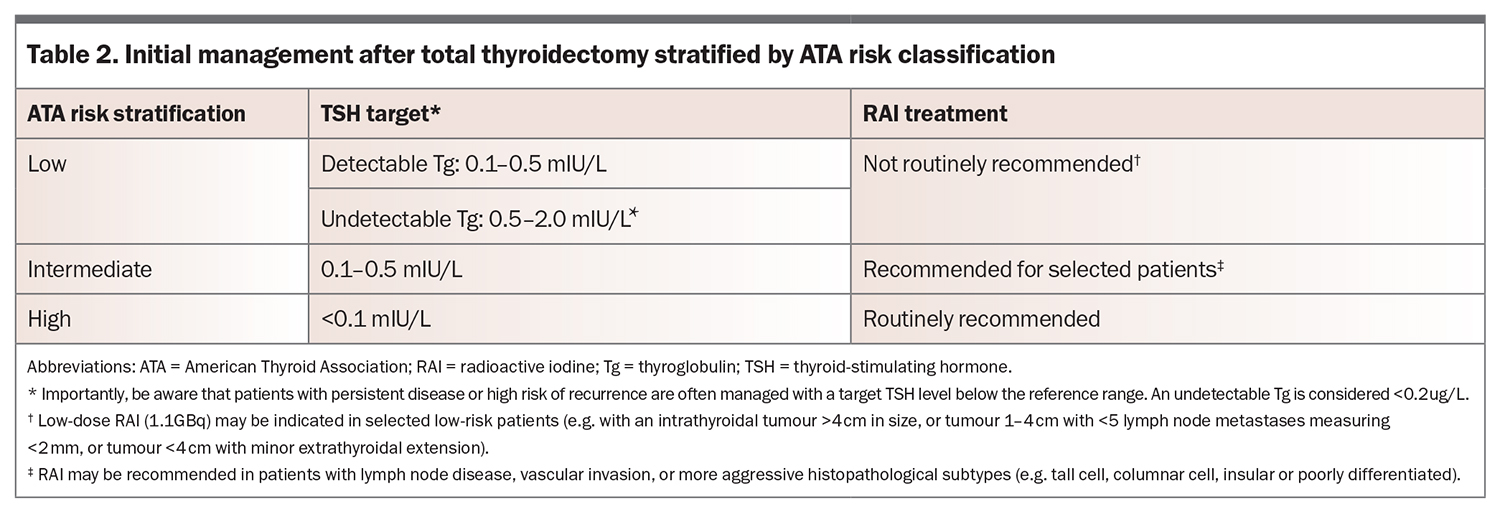
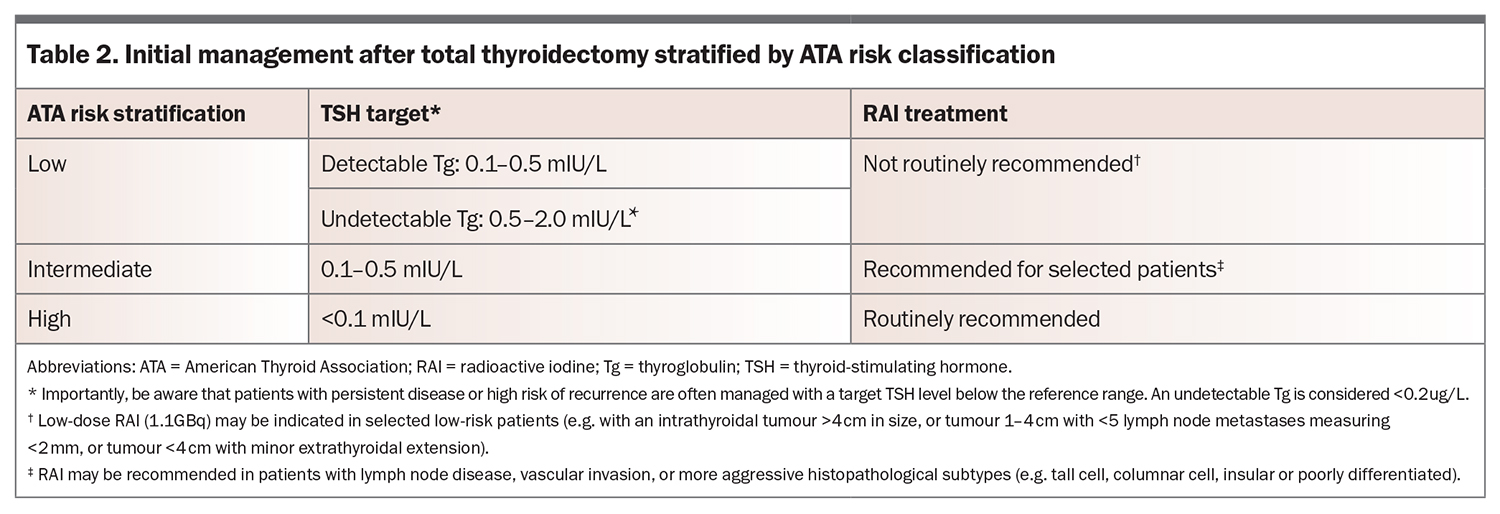
Postoperative management includes TSH suppression in most patients and RAI in high-risk and selected intermediate-risk patients. Management is based on the ATA risk and presence of detectable Tg and/or TgAb postoperatively.
Role of Tg and TgAb
Monitoring of serum Tg and TgAbs levels is an important postoperative tool to assess residual and recurrent DTC. Initially, Tg and TgAb levels should be measured four to six weeks postoperatively to assess for residual disease. The simultaneous measurement of TgAb and Tg levels is crucial, as TgAb can interfere with the accuracy of Tg measurement. Serial monitoring should occur at the same pathology provider to allow for accurate comparison.
Up to 25% of patients with DTC have detectable TgAb, particularly if there is concomitant Hashimoto’s thyroiditis. Following total thyroidectomy, Tg and TgAb should become undetectable. Rising or elevated Tg or TgAb levels are concerning for persistent or recurrent disease and require further investigation with neck ultrasound.9 Management recommendations are summarised in Table 2.
Radioactive iodine
If RAI is recommended, the dose will depend on the goal of treatment and will be guided by the treating endocrinologist. Recent trials have established noninferiority of either none or lower RAI doses for initial ablation in low- or intermediate-risk patients, respectively. Patients should be advised to consume a low-iodine diet for seven days before planned RAI treatment. RAI must be given with a stimulated TSH level above 30 mIU/L to maximise the uptake of iodine, which is usually achieved by the administration of recombinant TSH (thyrotropin alfa). Recombinant TSH administration may require GP involvement as it is typically given in two doses in the outpatient setting at 48 hours and 24 hours before planned RAI treatment. RAI treatment is then administered in the inpatient setting, where the patient stays in a lead-lined isolation room for a few days. Before discharge, a post-treatment whole-body iodine-131 uptake scan is performed to visualise normal or cancerous thyroid tissue remnant, thyroid cancer recurrence or distant metastases. For a few days following discharge, patients will be advised to take extra precautions, such as sleeping alone, washing clothes separately and washing hands thoroughly and often.
Dynamic risk stratification
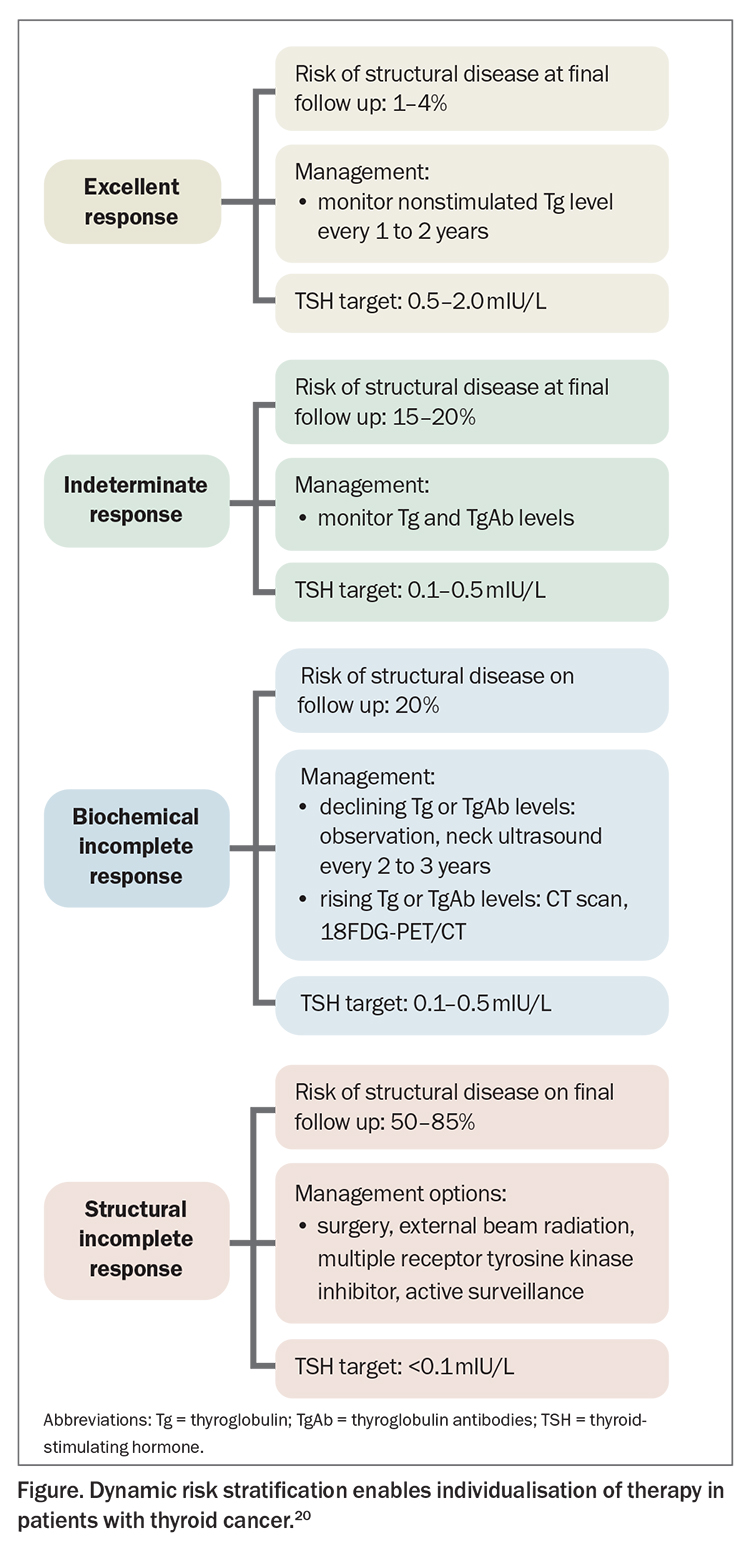
A key principle that has emerged in recent years is that of dynamic risk stratification to optimise individualised treatment.21,22 This is a dynamic, iterative tool used in patients with intermediate- and high-risk disease for six to 18 months post-total thyroidectomy to reclassify patient risk at each follow-up visit based on response to therapy, which is assessed by the trend of serum Tg, TgAb and imaging. Serial Tg and TgAb assessments should ideally occur on the same assay. At each visit, patients should be classified into one of the following outcomes:
- excellent response: no clinical, biochemical, or structural evidence of disease
- biochemical incomplete response: abnormal Tg or rising TgAb values in the absence of localisable disease
- structural incomplete response: persistent or newly identified locoregional or distant metastases
- indeterminate response: nonspecific biochemical or structural findings that cannot be confidently classified as either benign or malignant. This includes patients with stable or declining TgAb levels without definitive structural evidence of disease.
Reclassification at each follow-up visit enables individualisation and tailoring of management recommendations to the current clinical status, rather than relying on the initial risk stratification (Figure). This is important to avoid overtreatment in low-risk patients (with prolonged TSH suppression) and undertreatment in high-risk patients, and better correlates with long-term outcomes.
Radioiodine-refractory disease
Unfortunately, some patients with DTC stop responding to RAI as their tumours no longer trap iodine, making them refractory to RAI. This entity is called radioiodine-refractory (RAI-R) DTC and carries a 10-year survival rate of 10%. RAI-R disease is defined as DTC with lesions either never or no longer demonstrating RAI uptake on whole-body scans or progression of metastatic disease, despite substantial uptake of RAI during recent RAI treatment.9 RAI-R disease is a manifestation of de-differentiation of thyroid cancer cells such that they no longer express the sodium-iodide transporter and hence are unable to take up iodine. Overactivation of certain growth signalling pathways in thyroid cancer cells can predispose an individual to RAI-R disease, and this is an area of ongoing investigation.
Management options for RAI-R DTC are limited. Observation alone may be appropriate in asymptomatic slowly progressive disease. Surgery has a role in recurrent locoregional or low-volume distant metastases. External beam radiation therapy can also be considered for extensive local recurrence, particularly with laryngeal or tracheal invasion. Use of zoledronic acid or denosumab should be considered in patients with symptomatic or diffuse bone metastases to delay the time to occurrence of skeletal events (e.g. pathological fracture). Systemic treatments should be considered in patients with progressive or symptomatic disease that is not amenable to local treatment. However, conventional chemotherapy (e.g. doxorubicin) has limited efficacy and significant toxicity.9,23
Tyrosine kinase inhibitors: when are they indicated?
DTC cells overexpress vascular endothelial growth factor receptor, a tyrosine kinase receptor involved in tumour growth and angiogenesis. A phase 3, randomised, double-blind, multicentre study investigating the multiple receptor tyrosine kinase inhibitor, lenvatinib, showed significant improvements in progression-free survival (18.3 months in the lenvatinib group vs 3.6 months in the placebo group; p <0.001) and response rate (64.8% in the lenvatinib group vs 1.5% in the placebo group, p <0.001) in patients with progressive RAI-R DTC.24 Lenvatinib is TGA approved for the treatment of patients with progressive, locally advanced or metastatic RAI-R DTC and is also PBS listed (authority required) for this indication. Cabozantinib and sorafenib are other multiple tyrosine kinase inhibitors that are TGA approved (but not PBS listed) for RAI-R DTC.
Lenvatinib, similar to other multiple tyrosine kinase inhibitors, is associated with various ‘off target’ side effects, which necessitates dose reduction in most patients and treatment cessation in up to 20% of patients. The risk of treatment-induced hypertension is the greatest during the first two to three months; therefore, patients should undergo frequent blood pressure assessment (a home blood pressure monitor is recommended to facilitate this). Fatigue, gastrointestinal side effects and palmar-plantar dysaesthesia are also common.24-26 Liver enzymes and full blood count should be monitored during treatment because of the risk of elevation of alanine transaminase or aspartate transaminase levels, and cytopenia. Baseline and follow-up ECGs should also be performed given the risk of QTc prolongation. The use of lenvatinib can be associated with increased levothyroxine requirements; therefore, serum TSH levels should be monitored regularly, particularly because all patients taking lenvatinib for thyroid cancer are recommended for TSH suppression. Interestingly, the occurrence of treatment-induced hypertension and diarrhoea have been shown to be associated with better tumour response and survival in post-hoc analyses.26-28
Patients should be counselled appropriately by their treating endocrinologist on the benefits and risks of tyrosine kinase inhibitors before starting treatment, and informed of the alternative treatment approaches in a multidisciplinary team setting. Most patients taking multiple receptor tyrosine kinase inhibitors will ultimately experience disease progression and may be suitable for inclusion into certain clinical trials. Specialised somatic (tumour tissue) and germline (blood or saliva) genetic testing can identify patients with specific actionable mutations that may make them suitable for clinical trials using selective tyrosine kinase inhibitors. This approach should only be considered in specialised thyroid cancer centres.
There is emerging evidence on the use of selective tyrosine kinase inhibitors (e.g. combination of BRAF and MEK inhibitors) to ‘re-differentiate’ thyroid cancer cells, restoring their ability to take up iodine and sensitivity to radioactive iodine. Few small studies have described patients with RAI-R disease with return of radioiodine sensitivity after selective BRAF and MEK inhibition.23,25 Tyrosine kinase inhibitors are not currently TGA approved for this specific indication and further studies are ongoing.
Special considerations: pregnancy and fertility
If DTC is detected during pregnancy, thyroidectomy is preferably delayed to the postpartum period to reduce the risk of maternal and fetal complications. This is feasible due to the typically indolent nature of DTC. If surgery is delayed, women should be monitored with a thyroid ultrasound each trimester and TSH suppression is recommended to maintain TSH levels in the range of 0.3 to 2.0 mIU/L. If the cancer is deemed to be aggressive or rapidly growing, or the presence of nodal or distant metastases is identified, surgery is recommended in the second trimester to mitigate the risk of spontaneous abortion and pre-term labour in the first and third trimesters, respectively. Postoperative complications such as hypoparathyroidism and hypocalcaemia should be promptly managed to reduce maternal and fetal complications.
In women with a history of thyroid cancer, pregnancy should ideally be delayed for at least six (preferably 12) months following RAI, and RAI is contraindicated within three months of breastfeeding. On obtaining a positive pregnancy test, women should increase their levothyroxine dosing by 30 to 50% and have monthly TSH monitoring until 20 weeks’ gestation. This increase in dose is required because of an increase in serum thyroxine-binding globulin concentration, as a result of increased glycosylation and reduced hepatic clearance of thyroxine-binding globulin, which is mediated by oestrogen. Thereafter, second-monthly TSH testing can be performed. The target TSH level is below 2.5 mIU/L in the first and second trimester and below 3.0 mIU/L in the third trimester.29 Doses should be carefully downtitrated in the post-partum period.
RAI may lead to transient oligospermia in men and amenorrhoea in women. It is recommended that conception is delayed to six months after treatment. Men who have received high doses of RAI (cumulative doses >350 mCi) may not have full fertility restored until 12 months or more after treatment.30
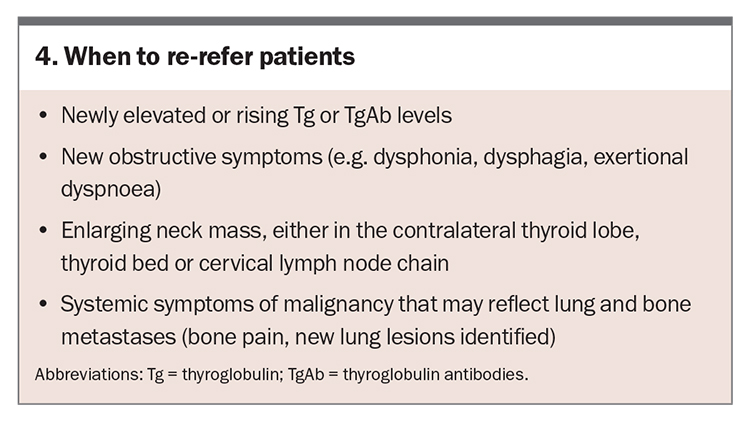
When to re-refer patients
The majority of DTC recurrence occurs within the first five years; however, late recurrence has been reported and necessitates long-term follow up. If there are concerning new symptoms or signs, consider re-referring the patient to an endocrinologist (Box 4).
Conclusion
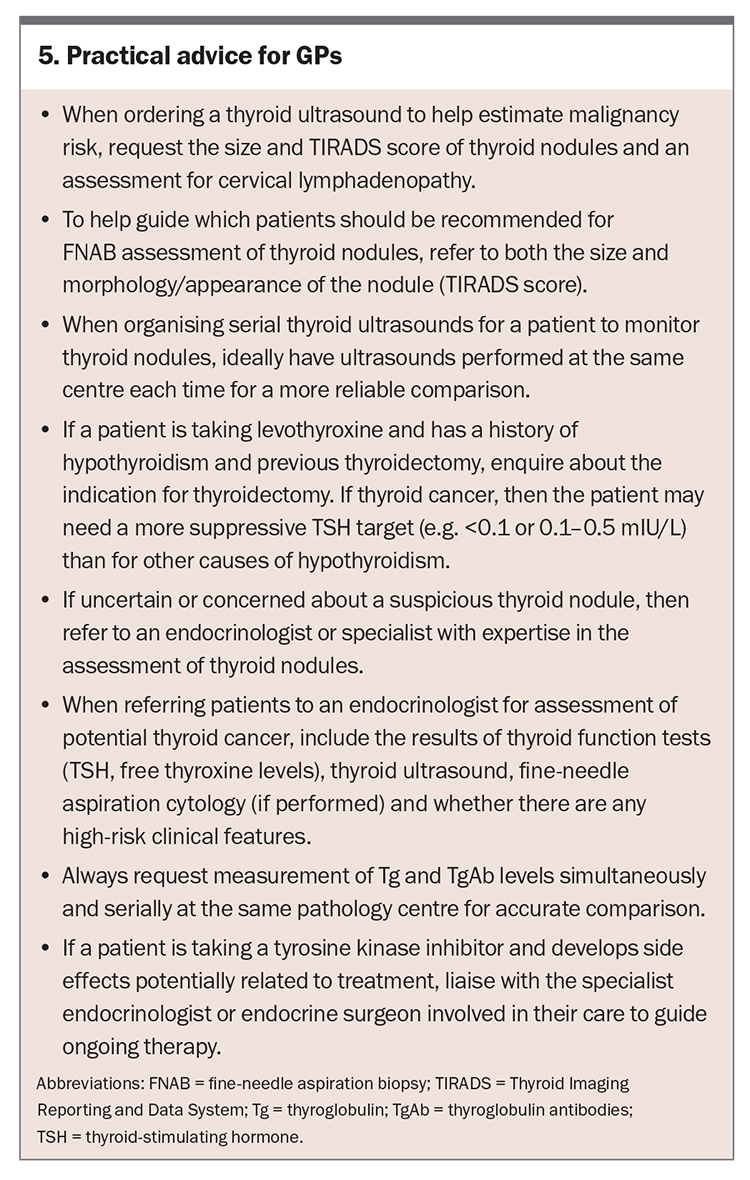
The incidence of DTC is on the rise, largely attributable to the increased incidental detection of thyroid nodules on ultrasound. Management of DTC should be tailored to the individual, and advanced disease may require multidisciplinary team input to stratify risk early and guide optimal management (for a summary of practical advice for GPs on the management of DTC, see Box 5). Following initial management, serial evaluation should involve dynamic risk stratification based on clinical assessment, Tg and TgAb levels and structural imaging, if indicated. Ultimately, the aim is to prioritise patient preference and goals of care, and to avoid the over treatment of low-risk disease and under treatment of high-risk disease. ET
COMPETING INTERESTS: None.
References
1. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017; 317: 1338-1348.
2. Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943-2008, before and after iodine supplementation. Int J Cancer 2012; 131: 2360-2366.
3. Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983-2007. Asia Pac J Public Health 2015; 2: NP223-229.
4. Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular and anaplastic: a morphological and epidemiological study. Endocr Pathol 2007; 18: 1-7.
5. Cancer Australia. Thyroid cancer in Australia statistics. Available online at: https://www.canceraustralia.gov.au/cancer-types/thyroid-cancer/statistics.
6. Australian Institute of Health and Welfare [Internet]. Cancer in Australia 2019: in brief. Available online at: https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2019-in-brief/summary.
7. Alexander EK, Cibas ES. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol 2022; 10: 533-539 (accessed October 2023)
8. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017; 14: 587-595.
9. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1-133.
10. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002; 87: 1941-1946.
11. Cibas ES, Ali SZ: The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol 2009; 132: 658-665.
12. Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1856-1883.
13. Carling T, Udelsman R. Thyroid cancer. Annu Rev Med 2014; 65: 125-137.
14. Tuttle RM. Controversial issues in thyroid cancer management. J Nucl Med 2018; 59: 1187-1194.
15. Cho JS, Shin SH, Song YJ, e al. Is it possible to predict hypothyroidism after thyroid lobectomy through thyrotropin, thyroglobulin, anti-thyroglobulin, anti-thyroglobulin, and anti-microsomal antibody? J Korean Surg Soc 2011; 81: 380-386.
16. Kim SY, Kim HJ, Kim SM, et al. Thyroid hormone supplementation therapy for differentiated thyroid cancer after lobectomy: 5 years of follow-up. Front Endocrinol 2020; 11: 520.
17. Tourani SS, Fleming B, Gundara J. Value of thyroglobulin post hemithyroidectomy for cancer: a literature review. ANZ J Surg 2021; 91: 724-729.
18. Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer – recent advances and future directions. Nat Rev Endocrinol 2018; 14: 670-683.
19. Tuttle M, Morris LF, Haugen B, et al. 2017. Thyroid-differentiated and anaplastic carcinoma. In: Amin MB, Edge SB, Greene F, et al (eds) AJCC Cancer Staging Manual. Eighth edition. Springer International Publishing, New York, New York.
20. Krajewska J, Chmielik E, Jarząb B. Dynamic risk stratification in the follow-up of thyroid cancer: what is still to be discovered in 2017? Endocr Relat Cancer 2017; 24: R387-R402.
21. Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: from detection to final follow-up. J Clin Endocrinol Metab 2019; 104: 4087-4100.
22. Pitoia F, Jerkovich F. Dynamic risk assessment in patients with differentiated thyroid cancer, endocrine-related cancer. 2019; 26: R553-R566.
23. Tumino D, Frasca F, Newbold K. Updates on the management of advanced, metastatic, and radioiodine refractory differentiated thyroid cancer. Front Endocrinol 2017; 8: 312.
24. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015; 372: 621-630.
25. Fallahi P, Ferrari SM, Galdiero MR, et al. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin Cancer Biol 2022; 79: 180-196.
26. Gild ML, Tsang VHM, Clifton-Bligh RJ, Robinson BG. Multikinase inhibitors in thyroid cancer: timing of targeted therapy. Nat Rev Endocrinol 2021; 17: 225-234.
27. Wirth LJ, Tahara M, Robinson B, et al. Treatment-emergent hypertension and efficacy in the phase 3 study of (E7080) Lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018; 124: 2365-2372.
28. Haddad RI, Schlumberger M, Wirth LJ, et al. Incidence and timing of common adverse events in lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine 2017; 56: 121-128.
29. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27:315.
30. Sawka AM, Lea J, Alshehri B, et al. A systematic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol (Oxf) 2008; 68: 610.