Heart failure in patients with diabetes: intersecting comorbidities and an evolving treatment landscape

Heart failure (HF) and diabetes are common, increasingly prevalent conditions among people in Australia, and are frequently encountered in primary care. The combination of HF and diabetes confers a poor prognosis, and management can be challenging. Sodium-glucose cotransporter-2 inhibitors and finerenone (a mineralocorticoid receptor antagonist) have recently altered the treatment landscape for both HF and diabetes, and provide important additions to the ‘therapeutic arsenal’ against cardio-renal-metabolic disease.
- Heart failure and diabetes are common and increasingly prevalent conditions that frequently coexist, making their management more complex.
- Heart failure confers a high mortality and morbidity. Patients with both heart failure and diabetes have a significantly poorer prognosis than those with either condition in isolation.
- Patients with heart failure with reduced ejection fraction should be treated with four classes of medications, unless intolerant or contraindicated: an angiotensin receptor neprilysin inhibitor or ACE inhibitor, a heart failure-specific beta blocker, a mineralocorticoid receptor antagonist (MRA) and a sodium-glucose cotransporter-2 (SGLT-2) inhibitor.
- Patients with heart failure with preserved ejection fraction should be considered for an SGLT-2 inhibitor, which is the only class of medication that has been shown to achieve significant improvements in cardiovascular mortality and heart failure-related hospitalisation.
- Patients with type 2 diabetes mellitus have high rates of heart failure, and preventative treatments are recommended. SGLT-2 inhibitors should be considered to reduce the risk of heart failure in all patients with type 2 diabetes, as well as finerenone (a third-generation MRA) to reduce the risk of heart failure, in patients with type 2 diabetes and macroalbuminuric chronic kidney disease.
- SGLT-2 inhibitors have multiple beneficial effects beyond achieving glycaemic control alone. They represent an important therapeutic advance in the management of cardio-renal-metabolic disease.
Heart failure (HF) and diabetes mellitus are commonly encountered comorbidities. HF affected about 511,000 adults in Australia (2.1%) in 2017, and this number is projected to grow to 657,000 by 2025.1 Diabetes affected an estimated 1.3 million people in Australia (5.3%) in 2022, with a rising prevalence.2 These increases are driven by an ageing population and the increasing prevalence of obesity, hypertension, hypercholesterolaemia, vascular disease and obstructive sleep apnoea. In patients with HF, about 30% also have diabetes, which is associated with higher rates of mortality, HF hospitalisation and poorer quality of life, compared with patients with HF without diabetes.3
Complex interaction between diabetes and heart failure
Patients with diabetes have a two- to fivefold increased risk of developing HF, compared with age-matched controls without diabetes.4-6 This higher incidence of HF in patients with diabetes persists after adjusting for age, hypertension, hypercholesterolaemia and coronary artery disease.
Multiple trials have explored the relationship between glycaemic control and cardiovascular outcomes, such as the Action in Diabetes and Vascular Disease-PreterAx and DiamicroN Controlled Evaluation (ADVANCE), Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, Veterans Affairs Diabetes Trial (VADT) and UK Prospective Diabetes Study.7-10 The findings of these trials suggested that improved glycaemic control did not translate to improved cardiovascular outcomes. A meta-analysis of 37,229 patients suggested that improved glycaemic control did not reduce the risk of HF or HF hospitalisation.11 The risk of cardiovascular events and HF in patients with diabetes is not related to hyperglycaemia alone but is conferred by multiple factors beyond glycaemic control.
Diabetes results in the dysregulation of neurohormonal, vascular and cellular mechanisms, which ultimately contributes to myocardial damage and HF.12-14 Neurohormonal changes include activation of the renin–angiotensin–aldosterone pathway, impaired cardiac sympathetic innervation and adrenergic hyperactivity. Microvascular dysfunction occurs because of endothelial-mediated impairment in coronary flow reserve, contributing to tissue hypoxia that can contribute to HF, through recurrent ischaemic insults. Cellular changes include increased oxidative stress, endoplasmic reticulum stress, changes in mitochondrial bioenergetics and substrate utilisation (with reduced myocardial glucose utilisation and increased fatty acid utilisation), accumulation of advanced glycated end-products, abnormalities in contractile proteins and impaired cellular relaxation.
Importantly, these biological mechanisms provide potential therapeutic targets in the treatment of HF and diabetes. Targeting these pathways may improve the prognosis and trajectory of diabetes and HF, beyond glycaemic control.
Prevalence and the Australian context
The prevalence of both HF and diabetes in Australia is increasing over time and will represent a substantial burden of disease and healthcare utilisation in the future.
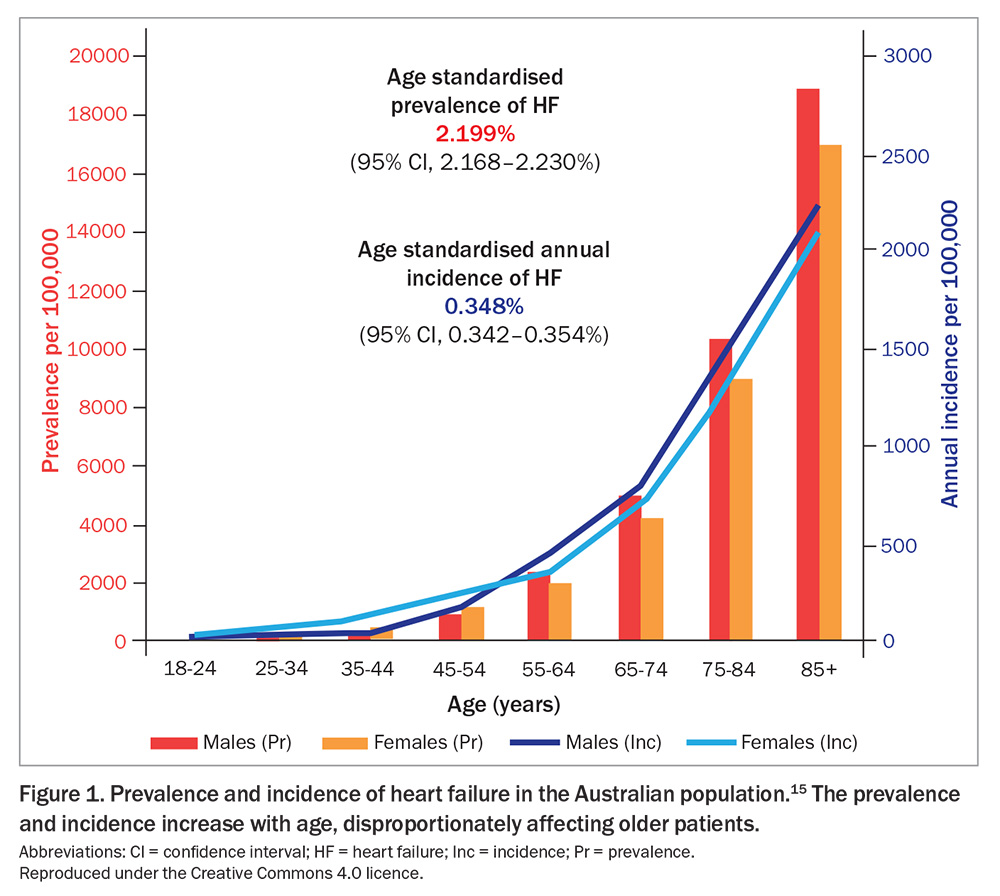
The prevalence of HF increases with age, disproportionately affecting older patients, with more than one-third of people affected being over the age of 75 years (Figure 1).15,16 This represents a substantial burden of healthcare utilisation within the primary care setting. Patients with HF visit their GPs 12.4 times a year on average and account for 3.6% of all patients presenting to GPs.17,18
Australian studies suggest that HF may be under-recognised in primary care, which may contribute to the lower use of guideline-directed medical therapy.16,19 Clinicians are encouraged to maintain a heightened HF awareness, particularly among older patients presenting for other health issues.
In patients with HF, an estimated 29% have comorbid diabetes or have a fourfold higher prevalence of type 2 diabetes compared with people without HF in the community setting.3,20 In patients hospitalised for decompensated HF, this number may be higher; an Australian cohort of HF admissions found that 49% had diabetes based on glycated haemoglobin (HbA1c) measurements.21 In patients with diabetes, an estimated 25% have comorbid HF or have a two- to fivefold risk of developing HF compared with people without diabetes.4,5,13
Myocardial dysfunction develops before clinical HF in patients with diabetes, involving dysregulated neurohormonal, vascular and cellular mechanisms associated with diabetes. Echocardiography studies have shown that asymptomatic systolic and/or diastolic dysfunction is detectable in up to 68% of patients who have had type 2 diabetes for five years without overt HF.22 Myocardial damage is, therefore, an early, undetected complication of diabetes. Diabetic cardiomyopathy describes this myocardial vulnerability. In patients with diabetes, independent risk factors for the development of HF include advanced age, greater duration of disease, insulin use and the presence of coronary artery disease or chronic kidney disease.23
Heart failure is a high morbidity condition, and with diabetes is worse
HF confers a high mortality and morbidity. Survival rates range from 81 to 91% at one year, and 52 to 63% at five years.18,24-26 Patients with HF with preserved ejection fraction (HFpEF) have slightly better survival compared with those with HF with reduced ejection fraction (HFrEF), although the difference is small.27 Effective management of HF is important, as survival is significantly decreased following any and each subsequent HF hospitalisation.28
Diabetes is the second most common major comorbidity in patients with HF (29%), following chronic kidney disease (41%).3 In patients with HF, the presence of diabetes is independently associated with a significant increase in all-cause mortality and HF hospitalisation, compared with HF patients without diabetes.3 Patients with HF and comorbid type 2 diabetes have a reduced median survival by 1.1 years (from 4.6 years to 3.5 years), compared with those without type 2 diabetes.29 In patients with diabetes, similarly, the presence of HF increases mortality, compared with patients without HF.30 This increased mortality remains after adjusting for other confounders. Therefore, the combination of HF and diabetes poses unique management challenges.
Medical therapy for HFrEF
The 2018 National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia strongly recommended three classes of medications to reduce mortality and HF hospitalisation in patients with HFrEF:
- ACE inhibitor, or angiotensin receptor blocker (ARB) if ACE inhibitors are contraindicated or not tolerated
- HF-specific beta blocker
- mineralocorticoid receptor antagonist (MRA).31
Since the publication of these guidelines, clinical trials evaluating novel therapies for HF have also shown improved outcomes, with benefits in mortality and HF hospitalisation. Subsequently, a consensus statement by Australian HF clinicians was published in 2022, providing updated recommendations.32
The additional benefit of neprilysin inhibition combined with renin–angiotensin system inhibition was shown in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study.33 This double-blinded, randomised controlled trial showed that the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril-valsartan significantly reduced mortality, reduced HF hospitalisation and improved HF symptoms, compared with the ACE inhibitor enalapril, with an even greater benefit seen in patients with coexistent type 2 diabetes. Post-hoc analyses identified the benefit of early initiation of sacubitril-valsartan within 30 days to reduce HF hospitalisation, thereby supporting its use earlier in the treatment pathway, along with the PIONEER-HF study findings, which showed benefit to early ARNI versus ACE inhibitor use.34-36 Consequently, it is strongly recommended that an ARNI should be considered first-line for HFrEF (and is preferred over an ACE inhibitor), provided it does not compromise the commencement of other first-line HFrEF therapies.32 If hypotension is a concern, then an upfront ACE inhibitor strategy may be considered with a switch to an ARNI once stabilised.
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors have also been evaluated in randomised controlled trials for patients with HFrEF (with or without type 2 diabetes), and have shown significant reductions in cardiovascular events, total mortality and HF hospitalisation, compared with placebo. The only two SGLT-2 inhibitors indicated and PBS listed for HF treatment in Australia are dapagliflozin and empagliflozin. In the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, use of dapagliflozin showed significant reductions in the composite primary endpoint of cardiovascular death or worsening HF, as well as significant reductions in both cardiovascular mortality and HF hospitalisation.37 In the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) trial, use of empagliflozin showed significant reductions in the composite primary endpoint of cardiovascular death or HF hospitalisation, driven by a significant reduction in HF hospitalisation.38 A meta-analysis combining the data from the DAPA-HF and EMPEROR-Reduced trials showed that SGLT-2 inhibitors significantly reduced the incidence of all-cause mortality, cardiovascular mortality, first hospitalisation for HF and first kidney composite event.39 Importantly, these benefits of SGLT-2 inhibitors were similar irrespective of the presence of diabetes. Consequently, it is strongly recommended that an SGLT-2 inhibitor (dapagliflozin or empagliflozin) is commenced in patients with HFrEF, regardless of the presence of diabetes.32
The Guidelines and consensus statement, when considered together, strongly recommend four classes of medications in all patients with HFrEF (unless contraindicated or intolerant): an ARNI (preferred, or an ACE inhibitor), HF-specific beta blocker, MRA and SGLT-2 inhibitor.31,32 This is consistent with recommendations made in recent European and American practice guidelines.40,41 A suggested treatment algorithm for initiating these four therapies is provided in the Flowchart.32
Medical therapy for HFpEF
Previously, medical therapy for HFpEF had not demonstrated robust evidence for benefit. Clinical trials for HFpEF have evaluated perindopril, candesartan, irbesartan, carvedilol, spironolactone, sacubitril- valsartan and digoxin, but these trials failed to show benefit.42-48 However, candesartan and spironolactone specifically showed reductions in HF hospitalisation, and can be considered in selected patients.41,43,46
Before 2021, no medication had shown a benefit in mortality in patients with HFpEF and, hence, management of HFpEF had focused on the relief of congestion and optimisation of comorbidities, particularly hypertension and atrial fibrillation.49
Subsequently, SGLT-2 inhibitors became the first class of medication to achieve significant improvements in primary outcomes in patients with HFpEF in clinical trials. Empagliflozin was shown in the EMPEROR-Preserved trial to significantly reduce cardiovascular death or HF hospitalisation by 21%, compared with placebo.51 Dapagliflozin was shown in the Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial to significantly reduce cardiovascular death or worsening HF by 18%, compared with placebo.50 These benefits were irrespective of the presence of type 2 diabetes, and baseline left ventricular ejection fraction. Consequently, it is recommended that an SGLT-2 inhibitor (dapagliflozin or empagliflozin) is considered in patients with HFpEF, regardless of the presence of diabetes.32
Medical therapy for type 2 diabetes
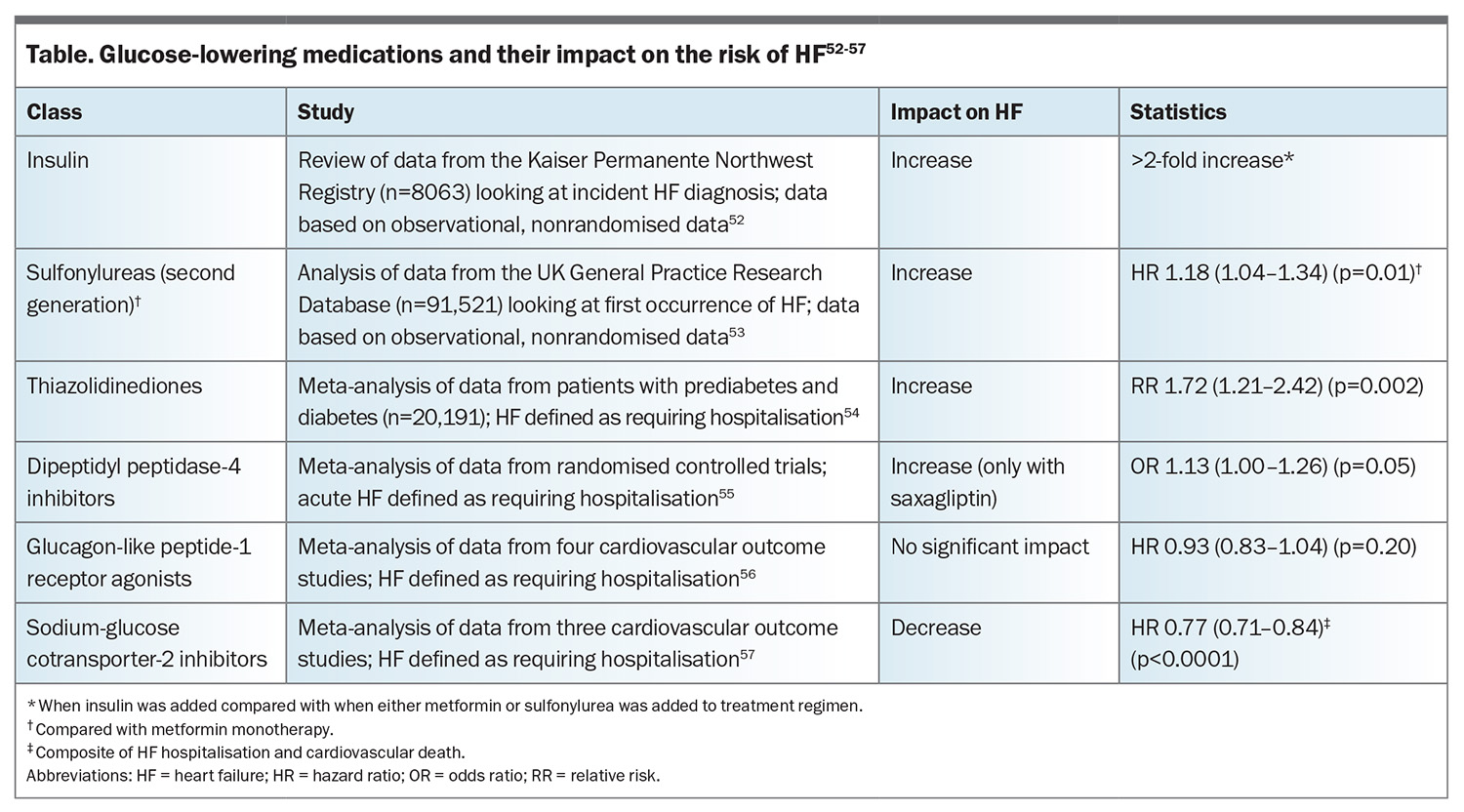
Glucose-lowering medications for diabetes may have variable effects on the risk of developing HF, which should be considered in the selection of glucose-lowering therapy (Table).52-57 Thiazolidinediones have been shown in a meta-analysis to significantly increase the risk of HF requiring hospitalisation.54 Similarly, dipeptidyl peptidase-4 inhibitors (predominantly saxagliptin) have been shown in a meta-analysis to increase the risk of HF requiring hospitalisation compared with no use, although the difference was borderline.55 Sulfonylureas and insulins may also increase the risk of developing HF, although these findings are based on observational data only.52,53 Metformin has been shown in some studies to reduce the risk of new-onset HF, although this is based on observational data only.52,58,59
Prevention of heart failure in patients with type 2 diabetes
Patients with type 2 diabetes are at a higher risk of developing HF. Studies have identified that SGLT-2 inhibitors and finerenone may be recommended to reduce the risk of developing HF in patients with type 2 diabetes.
Use of SGLT-2 inhibitors in patients with type 2 diabetes has shown consistent reductions in the development of cardiovascular disease, including reductions in HF and HF hospitalisation.57,60,64 Consequently, SGLT-2 inhibitors are recommended in patients with type 2 diabetes who are at high risk of cardiovascular disease, to decrease the risk of developing HF.32
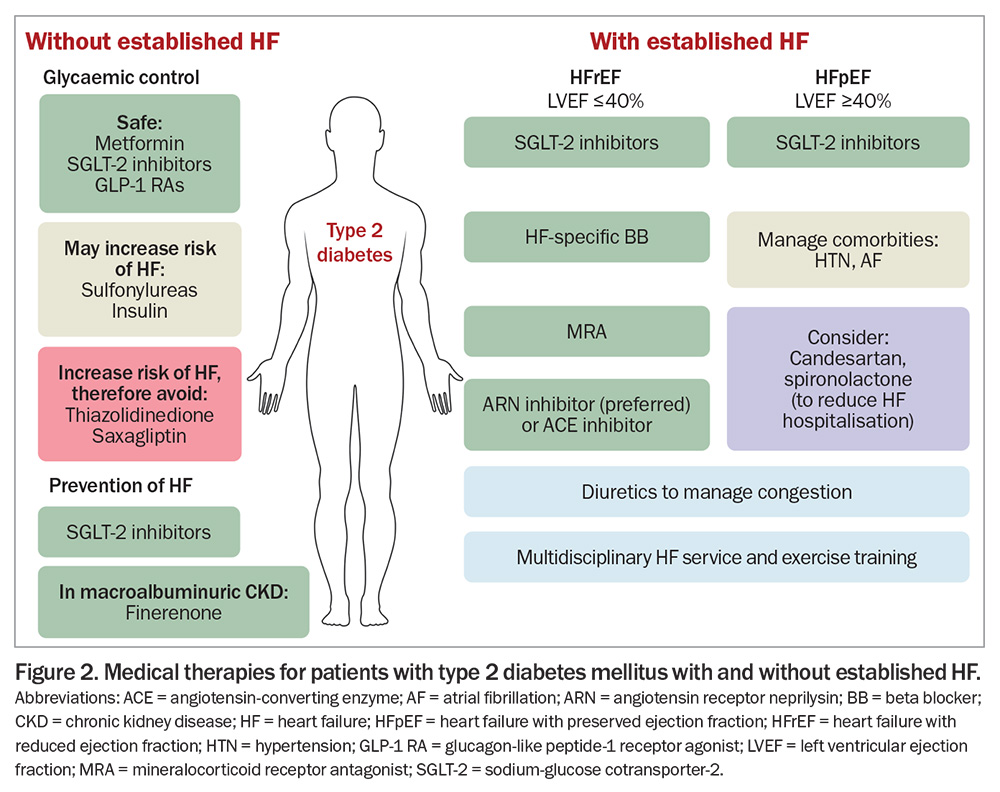
Finerenone is a selective, nonsteroidal MRA that has been evaluated in patients with type 2 diabetes and macroalbuminuric chronic kidney disease (estimated glomerular filtration rate [eGFR] greater than 25 mL/min/1.73 m2). The Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial showed that in patients with type 2 diabetes and macroalbuminuric chronic kidney disease, finerenone significantly reduced a composite cardiovascular primary endpoint (driven predominantly by decreased HF hospitalisation) and reduced new-onset HF.65,66 Consequently, finerenone should be considered to reduce the risk of developing HF in patients with type 2 diabetes and macroalbuminuric chronic kidney disease.32 In Australia, finerenone is PBS listed to delay the progressive decline of kidney function in patients with type 2 diabetes and macroalbuminuric diabetic kidney disease, in addition to standard of care, including ACE inhibitors or ARBs, and SGLT-2 inhibitors, unless contraindicated or intolerant. Medical therapies for patients with type 2 diabetes with or without HF are outlined in Figure 2.
SGLT-2 inhibitors
SGLT-2 inhibitors have emerged as important treatments in the ‘therapeutic arsenal’ for HF and diabetes. Evidence supports their use for:
- add-on therapy for glycaemic control in patients with type 2 diabetes
- a reduction in cardiovascular death in patients with type 2 diabetes and existing cardiovascular disease
- the prevention of HF in patients with type 2 diabetes and high cardiovascular risk
- the treatment of HFrEF, to decrease mortality and decrease HF hospitalisation (irrespective of the presence of diabetes)
- the treatment of HFpEF, to decrease mortality and decrease HF hospitalisation (irrespective of the presence of diabetes).
Mechanisms of benefit
The benefits of SGLT-2 inhibitors are related to their multiple mechanisms of action across the cardio-renal-metabolic systems, beyond glycaemic control.67-70 SGLT-2 inhibitors inhibit glucose resorption at the proximal tubules, causing glycosuria, which lowers glycaemia and reduces HbA1c levels by about 0.8%.71 The accompanying natriuretic and diuretic effect reduces tubuloglomerular feedback and intraglomerular pressure, contributing to nephroprotective effects. This early natriuresis with reduced plasma volume, reduced blood pressure, reduced afterload and improved vascular function all contribute to a beneficial effect in patients with HF. Glycosuria and caloric loss contribute to weight loss, increased insulin sensitivity, lipid metabolism and reduced lipotoxicity. There are also associated reductions in adipose-mediated inflammation and proinflammatory cytokines, a shift towards ketogenesis as the metabolic pathway for the heart and kidneys, reduced oxidative stress, increased erythropoietin levels, reduced uric acid levels and reduced accumulation of advanced glycation end-products. These complex and interconnected mechanisms contribute to the cardioprotective and renoprotective effects of SGLT-2 inhibitors.
Practical considerations
The recommended dosing of SGLT-2 inhibitors for HF is empagliflozin 10 mg daily, or dapagliflozin 10 mg daily, in keeping with the doses used in the placebo-controlled EMPEROR-Reduced, EMPEROR-Preserved, DAPA-HF and DELIVER trials.37,38,50,51 The main limitation to the use of SGLT-2 inhibitors is renal impairment, which is common in both HF and diabetes. Empagliflozin should not be commenced in patients with eGFR less than 20 mL/min/1.73 m2. Dapagliflozin should not be commenced in patients with eGFR less than 25 mL/min/1.73 m2. Once commenced at higher eGFRs, they can be continued, if tolerated, until the eGFR falls below 20 mL/min/1.73 m2, at which time consideration may be given to cessation, in consultation with a renal physician. On initiation of an SGLT-2 inhibitor, there is an expected initial reduction in the eGFR at four weeks (short-term ‘dip’) followed by longer-term renoprotective effects (‘eGFR preservation’).72 Studies have shown an initial ‘dip’ average of about 2 to 5 mL/min/1.73 m2, with about 30 to 50% of patients showing a decline in the eGFR of more than 10% from baseline.73,74 Patients who experience a more than 10% early decrease in the eGFR had significantly improved long-term outcomes, including a slower chronic rate of decline in eGFR in the long term, reiterating the importance to ‘stay the course’ and continue the SGLT-2 inhibitor through the initial eGFR decline.73
Although the glucose-lowering effects and renoprotective effects of SGLT-2 inhibitors are somewhat attenuated in patients with a lower eGFR, the cardioprotective effects of SGLT-2 inhibitors remained consistent across the spectrum of renal dysfunction, and reductions in HF hospitalisation may actually be accentuated in patients with a lower eGFR.57,75,76 Other risks are hypoglycaemia (predominantly if a patient is receiving another medication that is prone to causing hypoglycaemia) and mild hypotension, and low risks of diabetic ketoacidosis and Fournier’s gangrene. Patients should be advised to temporarily withhold SGLT-2 inhibitors for three days before receiving general anaesthesia, when unwell (particularly with respiratory or gastrointestinal infection) or when needing to fast for prolonged periods of time, and counselled regarding genitourinary hygiene. SGLT-2 inhibitors should not be used in patients with type 1 diabetes, as they increase the risk of diabetic ketoacidosis.77
Conclusion
HF and diabetes are frequently encountered conditions that carry a serious prognosis, with high mortality and morbidity. The interplay between diabetes and HF is complex. Diabetes confers an increased risk of HF beyond glycaemic control, involving the dysregulation of neurohormonal, vascular and cellular mechanisms. Newer agents, which target these pathophysiological mechanisms, have significantly altered the treatment landscape of cardio-renal-metabolic disease in the past decade, and their use will become increasingly prevalent. It is important that clinicians are familiar with the role, indications, side effects and management of these medications. ET
COMPETING INTERESTS: Dr Chan: None. Professor Sindone has received consulting fees from Inside Practice, Roche, Menarini and Vifor; has received payment or honoraria from Abbott, Amgen, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol Myers Squibb, CSL, Edwards, Eli Lilly, Glaxo Smith Kline, HealthEd, Inside Practice, Menarini, Merck Sharp and Dohm, Moderna, Mylan, National Cardiac Monitoring Incorporated, Novartis, Novo Nordisk, Otsuka, Pfizer, Roche, Sanofi, Servier and Vifor; has received support for attending meetings from Pharmacosmos, Vifor, Astra Zeneca, Boehringer Ingelheim and Novartis; is a member of advisory boards for Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL, Eli Lilly, Glaxo Smith Kline, Menarini, Merck Sharp and Dohm, Moderna, Mylan, National Cardiac Monitoring Incorporated, Novartis, Novo Nordisk, Otsuka, Pfizer, Roche, Sanofi, Servier and Vifor; is a member of the Writing Committee for the Cardiac Society / National Heart Foundation Guidelines for the Management of Heart Failure in Australia; holds stock shares in National Cardiac Monitoring and National Cardiac Monitoring Australia; and has received non-payment support from Novartis, CSL, Vifor and Mylan.
References
1. Chen L, Booley S, Keates AK, Stewart S. Snapshot of heart failure in Australia. Melbourne: Mary MacKillop Institute for Health Research, Australian Catholic University; 2017. Available online at: https://www.acu.edu.au/about-acu/news/2017/june/snapshot-of-heart-failure-in-australia (accessed May 2024).
2. Australian Bureau of Statistics (ABS). Diabetes. Canberra: ABS; 2022. Available online at: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/diabetes/latest-release#footnotes (accessed May 2024).
3. van Deursen VM, Urso R, Laroche C, et al. Co‐morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014; 1 6: 103-111.
4. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34: 29-34.
5. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA 1979; 241: 2035-2038.
6. Hoek AG, Dal Canto E, Wenker E, et al. Epidemiology of heart failure in diabetes: a disease in disguise. Diabetologia 2024: 67: 574-601.
7. Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560-2572.
8. Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545-2559.
9. Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129-139.
10. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood- glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853.
11. Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011; 162: 938-948.
12. Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus: impact of glucose-lowering agents, heart failure therapies, and novel therapeutic strategies. Circ Res 2019; 124: 121-141.
13. Rosano GM, Vitale C, Seferovic P. Heart failure in patients with diabetes mellitus. Card Fail Rev 2017; 3: 52.
14. Perrone-Filardi P, Paolillo S, Costanzo P, Savarese G, Trimarco B, Bonow RO. The role of metabolic syndrome in heart failure. Eur Heart J 2015; 36: 2630-2634.
15. Australian Institute of Health and Welfare (AIHW). Cardiovascular disease: Australian facts 2011. Canberra: AIHW; 2011. Available online at: https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/cardiovascular-disease-australian-facts-2011/summary (accessed May 2024).
16. Sindone AP, Haikerwal D, Audehm RG, et al. Clinical characteristics of people with heart failure in Australian general practice: results from a retrospective cohort study. ESC Heart Fail 2021; 8: 4497-4505.
17. Britt H, Miller GC, Henderson J, et al. General practice activity in Australia 2014–15. Sydney: Sydney University Press; 2015.
18. Taylor CJ, Valenti L, Britt H, et al. Management of chronic heart failure in general practice in Australia. Aust Fam Phys 2016; 45: 823-827.
19. Parsons RW, Liew D, Neville AM, et al. The epidemiology of heart failure in the general Australian community-study of heart failure in the Australian primary care setting (SHAPE): methods. BMC Pub Health 2020; 20: 1-1.
20. Maack C, Lehrke M, Backs J, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association–European Society of Cardiology. Eur Heart J 2018;39: 4243-4254.
21. Khoo K, Lew J, Neef P, et al. Routine use of HbA1c amongst inpatients hospitalised with decompensated heart failure and the association of dysglycaemia with outcomes. Sci Rep 2018; 8: 13564.
22. Faden G, Faganello G, De Feo S, et al. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: data from the SHORTWAVE study. Diabetes Res Clin Pract 2013; 101: 309-316.
23. Wang Y, Negishi T, Negishi K, Marwick TH. Prediction of heart failure in patients with type 2 diabetes mellitus—A systematic review and meta-analysis. Diabetes Res Clin Pract 2015; 108: 55-66.
24. Blackledge HM, Tomlinson J, Squire IB. Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001. Heart 2003; 89: 615.
25. Parenica J, Spinar J, Vitovec J, et al. Long-term survival following acute heart failure: the Acute Heart Failure Database Main registry (AHEAD Main). Eur J Intern Med 2013; 24: 151-160.
26. Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S, Goto D, Takeshita A; JCARE-GENERAL Investigators. Characteristics and outcomes of patients with heart failure in general practices and Hospitals Japanese Cardiac Registry of Heart Failure in General Practice (JCARE-GENERAL). Circulation 2007; 71: 449-454.
27. Abebe TB, Gebreyohannes EA, Tefera YG, Abegaz TM. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: a retrospective cohort study. BMC Cardiovasc Disord 2016; 16: 1-8.
28. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007; 154: 260-266.
29. Johansson I, Edner M, Dahlström U, Näsman P, Rydén L, Norhammar A. Is the prognosis in patients with diabetes and heart failure a matter of unsatisfactory management? An observational study from the Swedish Heart Failure Registry. Eur J Heart Fail 2014; 16: 409-418.
30. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff Jr DC. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699-703.
31. Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of heart failure 2018. Med J Aust 2018; 209: 363-369.
32. Sindone AP, De Pasquale C, Amerena J, et al. Consensus statement on the current pharmacological prevention and management of heart failure. Med J Aust 2022; 217: 212-217.
33. McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New Engl J Med 2014; 371: 993-1004.
34. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54-61.
35. Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol 2016; 68: 241-248.
36. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. New Engl J Med 2019; 380: 539-548.
37. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995-2008.
38. Packer M, Anker SD, Butler J, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-reduced trial. J Am Coll Cardiol 2021; 77: 1381-1392.
39. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020; 396: 819-829.
40. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022; 24: 4-131.
41. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022; 79: e263-e421.
42. Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27: 2338-2345.
43. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003; 362: 777-781.
44. Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2011; 4: 324-331.
45. Yamamoto K, Origasa H, Hori M; J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013; 15: 110-118.
46. Selvaraj S, Claggett B, Shah SJ, et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail 2018; 20: 483-490.
47. McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020; 141: 338-351.
48. Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation 2006; 114: 397-403.
49. Jeffries A, Chan WPA. Heart failure with preserved ejection fraction: advances in management. Medicine Today 2023; 24: 21-27.
50. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. New Engl J Med 2021; 385: 1451-1461.
51. Solomon SD, McMurray JJ, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. New Engl J Med 2022; 387: 1089-1098.
52. Nichols GA, Koro CE, Gullion CM, Ephross SA, Brown JB. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev 2005; 21: 51-57.
53. Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009; 339: b4731.
54. Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007; 370: 1129-1136.
55. Li L, Li S, Deng K, et al. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ 2016; 352: i610.
56. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018; 6: 105-113.
57. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31-39.
58. Pantalone KM, Kattan MW, Yu C, et al. The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta diabetologica 2009; 46: 145-154.
59. Tseng CH. Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: a retrospective cohort analysis. J Am Heart Assoc 2019; 8: e011640.
60. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New Engl J Med 2019; 380: 2295-2306.
61. Wiviott SD, Berg DD. SGLT2 inhibitors reduce heart failure hospitalization and cardiovascular death: clarity and consistency. J Am Coll Cardiol 2023; 81: 2388-2390.
62. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. New Engl J Med 2020; 383: 1425-1435.
63. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021; 6: 148-158.
64. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New Engl J Med 2019; 380: 347-357.
65. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. New Engl J Med 2021; 385: 2252-2263.
66. Filippatos G, Anker SD, Pitt B, et al. Finerenone and heart failure outcomes by kidney function/albuminuria in chronic kidney disease and diabetes. Heart Fail 2022; 10: 860-870.
67. Marassi M, Fadini GP. The cardio-renal-metabolic connection: a review of the evidence. Cardiovasc Diabetol 2023; 22: 195.
68. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020; 17: 761-772.
69. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018; 61: 2108-2117.
70. Fonseca-Correa JI, Correa-Rotter R. Sodium-glucose cotransporter 2 inhibitors mechanisms of action: a review. Front Med 2021; 8: 777861.
71. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159: 262-274.
72. Umanath K, Testani JM, Lewis JB. "Dip" in eGFR: stay the course with SGLT-2 inhibition. Circulation 2022; 146: 463-465.
73. Adamson C, Docherty KF, Heerspink HJ, et al. Initial decline (dip) in estimated glomerular filtration rate after initiation of dapagliflozin in patients with heart failure and reduced ejection fraction: insights from DAPA-HF. Circulation 2022; 146: 438-449.
74. Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021; 99: 750-762.
75. Cherney DZ, Cooper ME, Tikkanen I, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int 2018; 93: 231-244.
76. Neuen BL, Young T, Heerspink HJ, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7: 845-854.
77. Hropot T, Battelino T, Dovc K. Sodium-glucose co-transporter-2 inhibitors in type 1 diabetes: a scoping review. Hormone Res Paediatr 2023; 96: 620-630.