Primary adrenal insufficiency: low-stress management

Primary adrenal insufficiency (Addison’s disease) is a life-threatening condition that is increasing in prevalence in Western countries. It is easily missed because of the nonspecific nature of its symptoms. Effective management is available that can restore wellbeing and minimise the risk of adrenal crisis in patients with this condition. This may be best delivered in a collaborative care model.
- Primary adrenal insufficiency (PAI), defined as cortisol insufficiency due to adrenal pathology, is often missed or the diagnosis is delayed because of its nonspecific symptoms.
- An adrenal crisis may be the presenting feature of adrenal insufficiency; precipitants include infection, surgery and medication nonadherence.
- Patients with a suspected adrenal crisis require urgent hydrocortisone treatment; pretreatment measurement of serum cortisol level may be useful, but treatment should not be delayed while awaiting test results.
- PAI can be definitively diagnosed using an adrenocorticotrophic hormone (Synacthen) stimulation test; chronic secondary (pituitary) adrenal insufficiency can also be diagnosed with this test.
- Patient education is pivotal for preventing adrenal crises, although complete elimination of these episodes does not appear possible with current measures.
The development of steroid hormone replacement for adrenal insufficiency from the 1950s was a triumph in medicine and led to near normalisation of survival in many patients with primary adrenal insufficiency (PAI), also known as Addison’s disease. However, residual clinical challenges in patients with PAI include impaired wellbeing in a substantial subset; the continuing risk of adrenal crisis; and uncertainty about optimal glucocorticoid dosing. Although PAI is rare, with a reported prevalence of 100 to 140 cases per million in Western countries, it is increasing in prevalence, particularly in women.1-4
This article reviews the presentation of people with PAI, their diagnosis and management, including treatment and prevention of adrenal crises.
What is primary adrenal insufficiency?
Adrenal insufficiency is defined as insufficient secretion of glucocorticoids from the adrenal glands to maintain homeostasis. Adrenal insufficiency can be primary, caused by direct adrenal pathology, or secondary, which implies deficient pituitary secretion of adrenocorticotrophic hormone (ACTH). The latter is often the result of pituitary disease or of hypothalamic disease and consequent insufficient secretion of corticotrophin-releasing hormone. Hypothalamic-origin adrenal insufficiency may also be termed tertiary adrenal insufficiency.
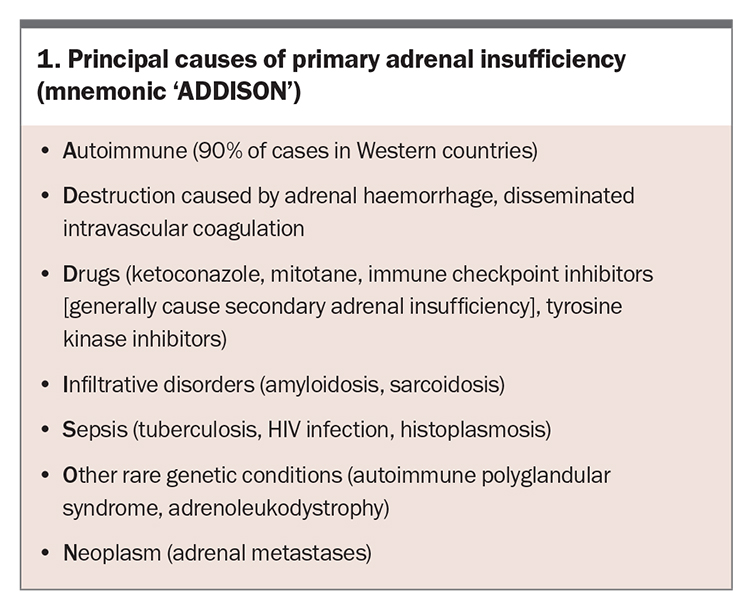
PAI was named Addison’s disease following Thomas Addison’s autopsy-based description with clinicopathological correlation in 1855.5 In addition to cortisol deficiency, PAI may be associated with impaired aldosterone production, with the subsequent volume contraction sensed by renal distal tubular macula densa cells, leading to increased release of the enzyme renin from afferent arteriolar juxtaglomerular cells.6 The causes of PAI are sometimes described by the mnemonic ‘ADDISON’ (Box 1). The most common cause in Western countries is autoimmune disease.
Secondary adrenal insufficiency involves cortisol deficiency and requires the same glucocorticoid replacement as PAI. However, it does not generally lead to aldosterone deficiency (as aldosterone is controlled by renin) or hyperpigmentation (which is a feature of ACTH hypersecretion).
Manifestations of primary adrenal insufficiency
The manifestations of PAI are due to the direct and indirect effects of cortisol and aldosterone deficiency. Hypocortisolism results in loss of the normal suppression of inflammatory cytokines, with consequent malaise, anorexia, body aches and fever.6,7 As a result of mineralocorticoid deficiency, patients may have salt craving, hyponatraemia, hyperkalaemia, tachycardia or hypotension. In some cases, the aetiology of PAI may also influence the clinical features, such as in cases of autoimmune disease or malignancy treated with immune checkpoint inhibitors.
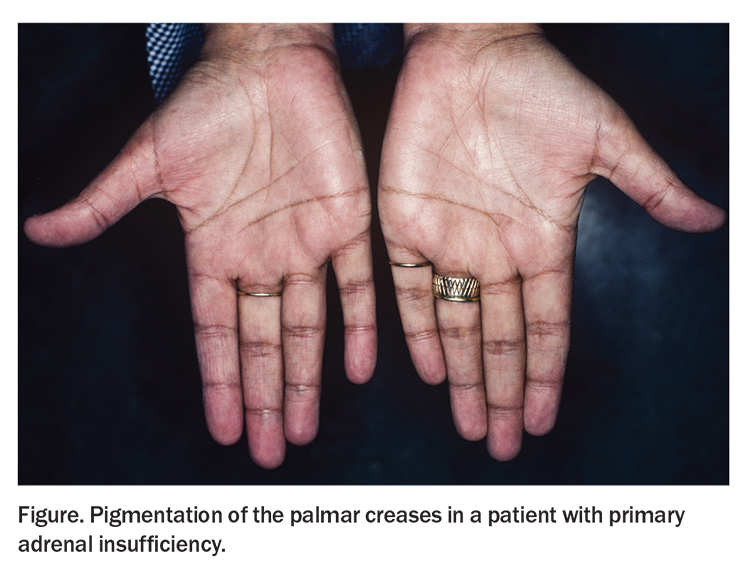
Hyperpigmentation of the skin, especially the palmar creases, nipples, nail beds and mucous membranes, results from enhanced secretion of melanocyte-stimulating hormone (MSH) (Figure).8 This occurs because MSH and ACTH share the same precursor, and decreased cortisol feedback enhances secretion of both.
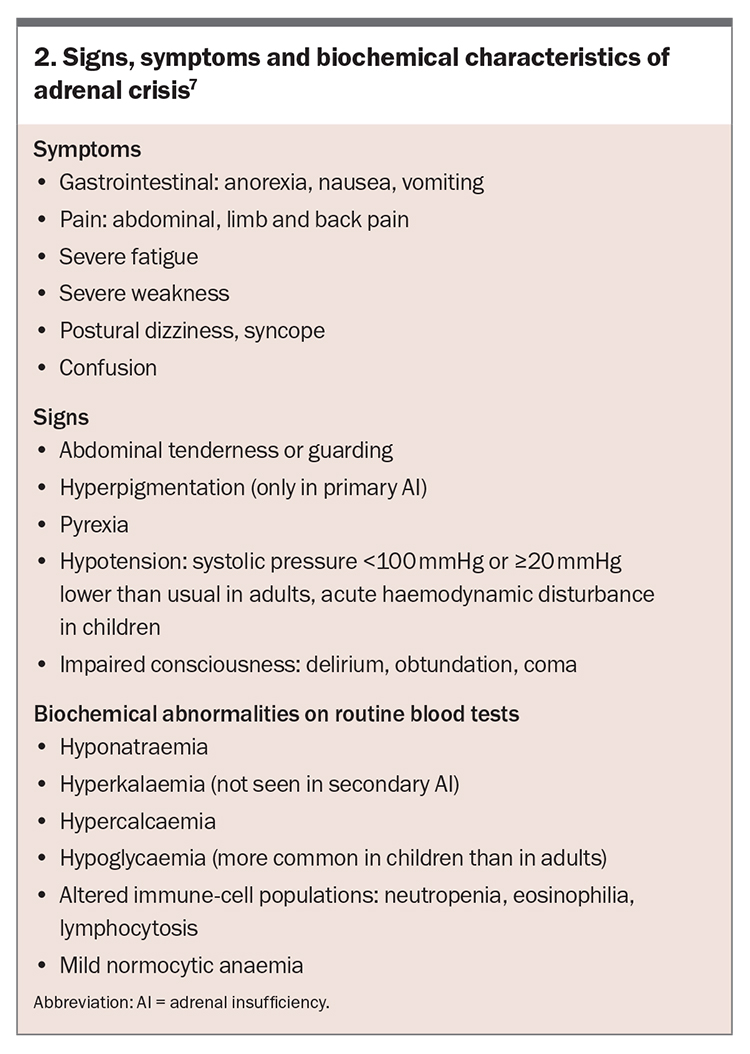
The nonspecific nature of the symptoms of hypocortisolism often delays diagnosis, leading to patients presenting with life-threatening adrenal (‘addisonian’) crisis.9 Features of adrenal crisis are detailed in Box 2.7 Diagnosis of an adrenal crisis can be complicated by the lack of a universally accepted definition.10 A recommended definition of adrenal crisis is hypotension, either absolute hypotension (systolic blood pressure less than 100 mmHg) or relative hypotension (reduction in systolic blood pressure of 20 mmHg or more), that improves markedly after administration of parenteral glucocorticoid therapy.7
Diagnosis of PAI
Who should be tested for PAI?
Patients with otherwise unexplained symptoms or signs that are suggestive of PAI should undergo an ACTH stimulation test when possible.6 However, in patients with severe adrenal insufficiency symptoms or an adrenal crisis, immediate therapy with intravenous hydrocortisone at an appropriate dose is recommended before an ACTH stimulation test, although measurement of plasma cortisol level may be useful.
Investigations for PAI
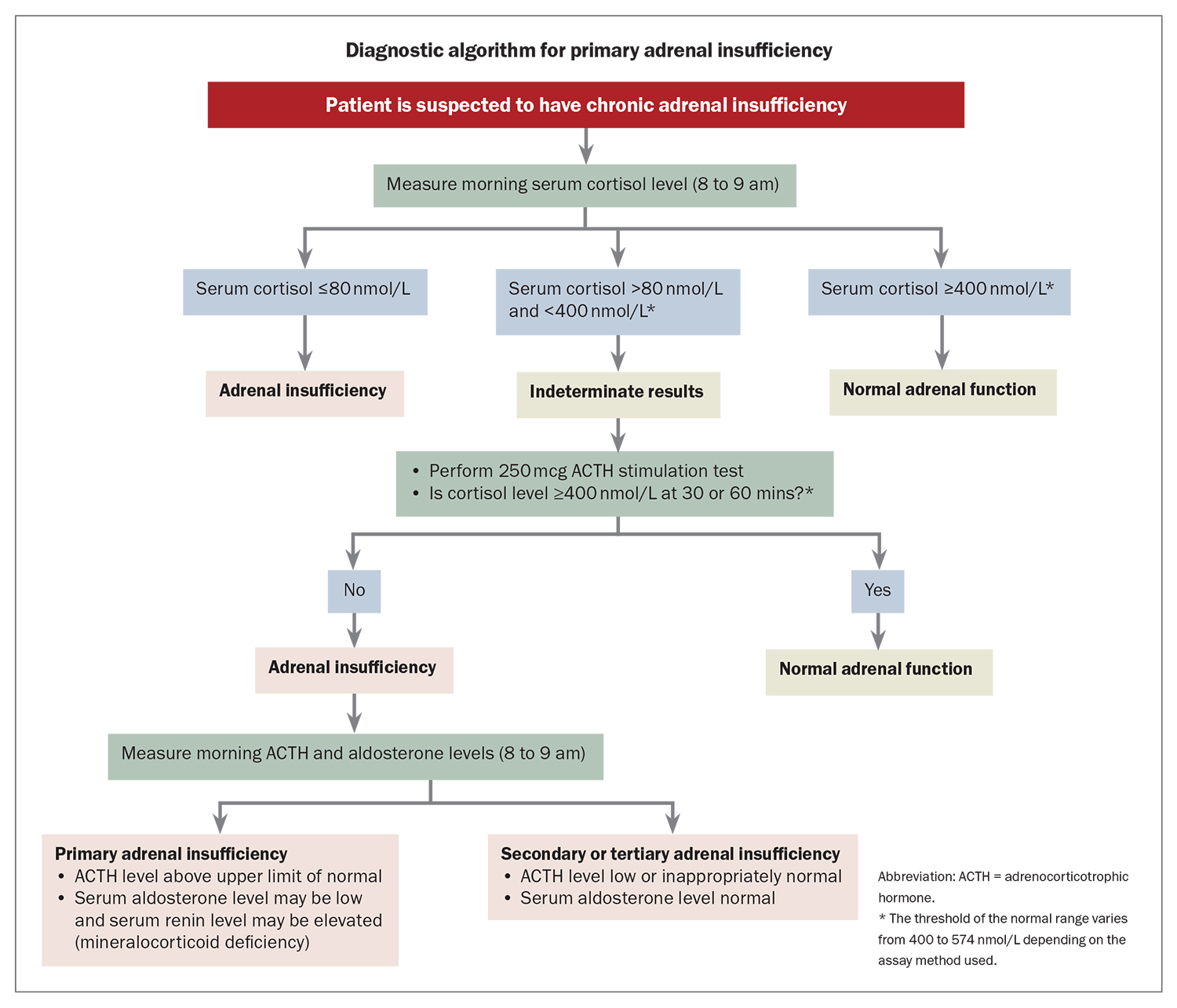
A diagnostic algorithm for PAI is shown in the Flowchart.
Cortisol measurement
In patients with suspected adrenal insufficiency, the first step may be a random or morning measurement of plasma cortisol level. Cortisol measurements are assay dependent, with recent use of more specific antibodies in commercial immunoassays and mass spectrometry yielding lower cortisol measurements.11 A plasma cortisol level greater than 400 to 574 nmol/L, depending on the assay method used, will exclude adrenal insufficiency.12 However, an ACTH stimulation test is required if there is ongoing clinical suspicion.6,13-15
A morning cortisol measurement is likely to be more definitive than a random measurement, as cortisol levels are at their physiological diurnal peak around the time of waking and at a nadir around midnight in people with a typical wake-sleep cycle. A morning cortisol level of 80 nmol/L or less suggests adrenal insufficiency, whereas a level between 80 and 400 to 574 nmol/L, depending on the assay method, is indeterminate.
Short tetracosactide (Synacthen) stimulation test
Tetracosactide (Synacthen), also known as cosyntropin, is a truncated synthetic ACTH peptide used in the ACTH simulation test. In the short Synacthen test (SST), baseline measurements of cortisol and ACTH levels are first obtained.6 A standard dose of Synacthen is then given intravenously or intramuscularly (250 mcg for adults and children aged 2 years and over, 125 mcg for children aged 1 to 2 years and 15 mcg/kg for infants aged 0 to 1 year). Blood samples are collected after 30 and 60 minutes or 60 minutes alone for measurement of cortisol and ACTH levels.6,15 Importantly, blood for measurement of ACTH level should be collected in an iced plastic or siliconised glass lavender-top EDTA (also known as edetic acid) tube.
In the SST, a peak cortisol level below 400 nmol/L at 30 or 60 minutes after Synacthen administration is consistent with adrenal insufficiency, allowing for variation in measurement as described above. Adrenal insufficiency is excluded if an appropriately elevated cortisol level is obtained (above a threshold of 400 to 574 nmol/L depending on the assay used).11 In healthy individuals, the cortisol level is lower at 30 minutes than at 60 minutes, and time-specific cut offs should be used.
Other assessments
If an SST is not feasible then PAI is suggested by the combination of a morning cortisol level less than 140 nmol/L and an elevated ACTH level (more than twice the upper limit of the reference range), allowing for the clinical circumstances. This also assumes no recent use of undetectable exogenous glucocorticoids. These include dexamethasone, which does not cross react, and glucocorticoids with short half-lives in plasma such as hydrocortisone (half-life of 90 minutes) and prednisolone (half-life of 150 minutes).
Patients with chronic PAI will also develop mineralocorticoid deficiency, manifesting as hyponatraemia, intravascular volume depletion and hyperkalaemia. Typically, plasma aldosterone level is low and renin level is markedly elevated.
Treatment of PAI
Glucocorticoid replacement therapy
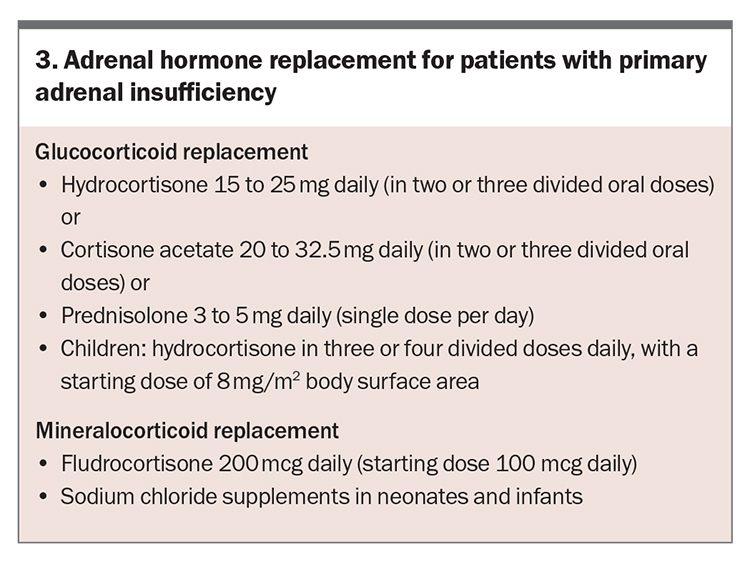
All patients with PAI should be treated with glucocorticoid replacement therapy.6 Dose requirements vary widely, even when corrected for body weight, because of variation in cortisol metabolism and glucocorticoid tissue sensitivity.16 There is no blood test to assess glucocorticoid dose adequacy. Monitoring of treatment response is by assessment of the patient’s body weight, postural blood pressure, energy levels and signs of excess glucocorticoid, such as centripetal weight gain.
The usual total daily glucocorticoid dose is 0.2 to 0.3 mg/kg hydrocortisone or equivalent; however, some patients require a higher dose to avoid recurrent symptoms of adrenal insufficiency and other patients require a lower dose to avoid features of glucocorticoid excess.16-19 Suggested replacement doses are hydrocortisone 15 to 25 mg daily or cortisone acetate 20 to 32.5 mg daily, in two or three divided oral doses (Box 3). The highest dose is given in the morning at awakening. The next dose is given either in the early afternoon (two hours after lunch) for the two-dose regimen or at lunch with a third dose in the afternoon for the three-dose regimen.
Prednisolone can be used as an alternative to hydrocortisone, usually at a single daily dose of 3 to 5 mg.6 Dexamethasone is not routinely recommended because of its lack of mineralocorticoid activity, potential to cross the placenta without inactivation and the absence of low-dose preparations required in PAI (0.3 to 0.4 mg), leading to a risk of Cushingoid adverse effects.6
Mineralocorticoid replacement therapy
Patients with features of mineralocorticoid deficiency should be started on fludrocortisone at a typical dose of 100 mcg in both adults and children. This can be uptitrated to 200 mcg daily. Salt intake should not be restricted. Response to treatment is monitored by clinical review, including assessment for salt craving and postural hypotension. Serum electrolyte and plasma renin concentrations can be used to titrate the dose of fludrocortisone. Early treatment of PAI with fludrocortisone may lead to clinically significant excess fluid retention caused by mineralocorticoid hypersensitivity, requiring a dose reduction and subsequent gradual uptitration with resolution of the oedema (which is generally in the lower limbs).
Patients with PAI may develop essential hypertension while taking fludrocortisone. In these circumstances, the dose of glucocorticoid replacement therapy should be assessed to ensure it is optimal, as supraphysiological glucocorticoid levels can also lead to hypertension. The target plasma renin level should be at the upper limit of the reference range. If the plasma renin level is markedly elevated despite a usual fludrocortisone dose then renovascular hypertension should be excluded with medical imaging. If the plasma renin level is low-normal or suppressed, or there are features of mineralocorticoid (fludrocortisone) excess, such as oedema or hypokalaemia, then the fludrocortisone dose should be reduced, generally by 50%.19 If hypertension persists despite optimisation of the fludrocortisone dose, antihypertensive therapy can be used. A calcium channel blocker is often effective.19
Treatment in pregnancy
Patients with PAI who are pregnant should be reviewed for clinical symptoms and signs of glucocorticoid excess or deficiency at least once per trimester. No prospective trials are available of PAI treatment in pregnancy, but pregnancy may lead to adrenal insufficiency symptoms that are difficult to differentiate from the effects of pregnancy alone, particularly postural hypotension, fatigue and nausea. Typically, glucocorticoid dose increases of about one-third are required in mid-pregnancy.6
Adrenal crisis
Recognising precipitants of adrenal crisis
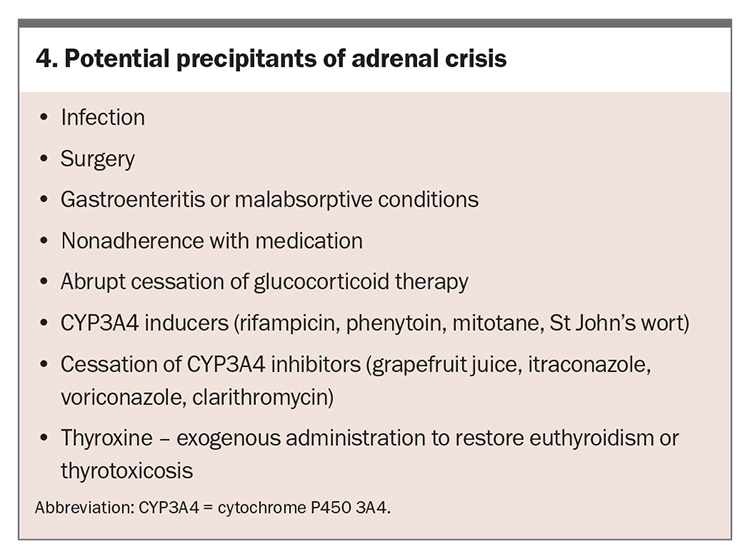
In patients with adrenal insufficiency, early recognition of an adrenal crisis is essential, given its high mortality.20 Potential precipitants of adrenal crisis are listed in Box 4.
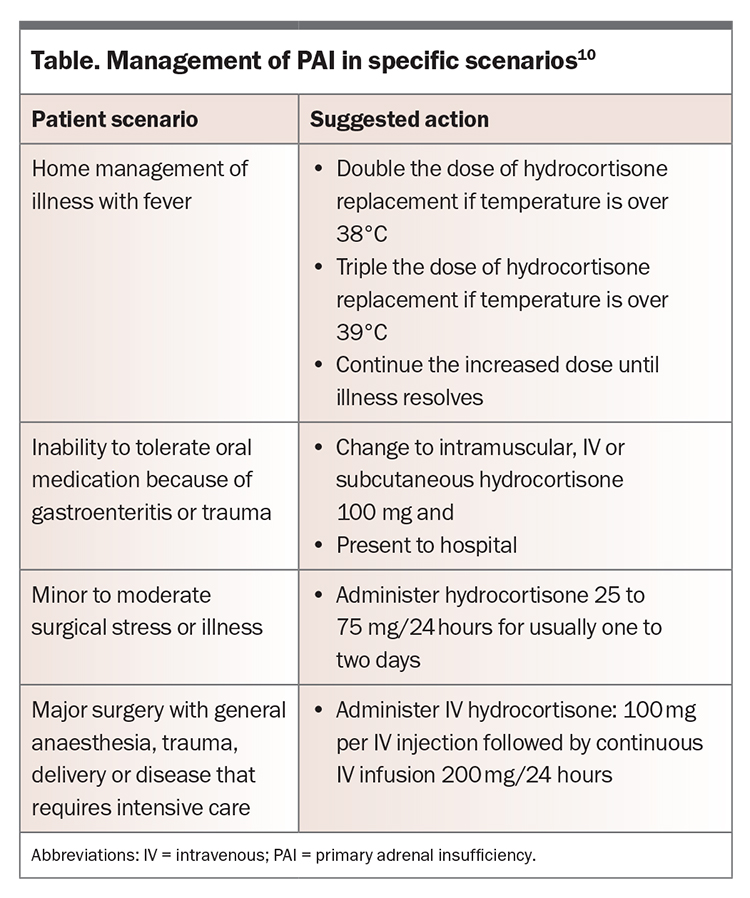
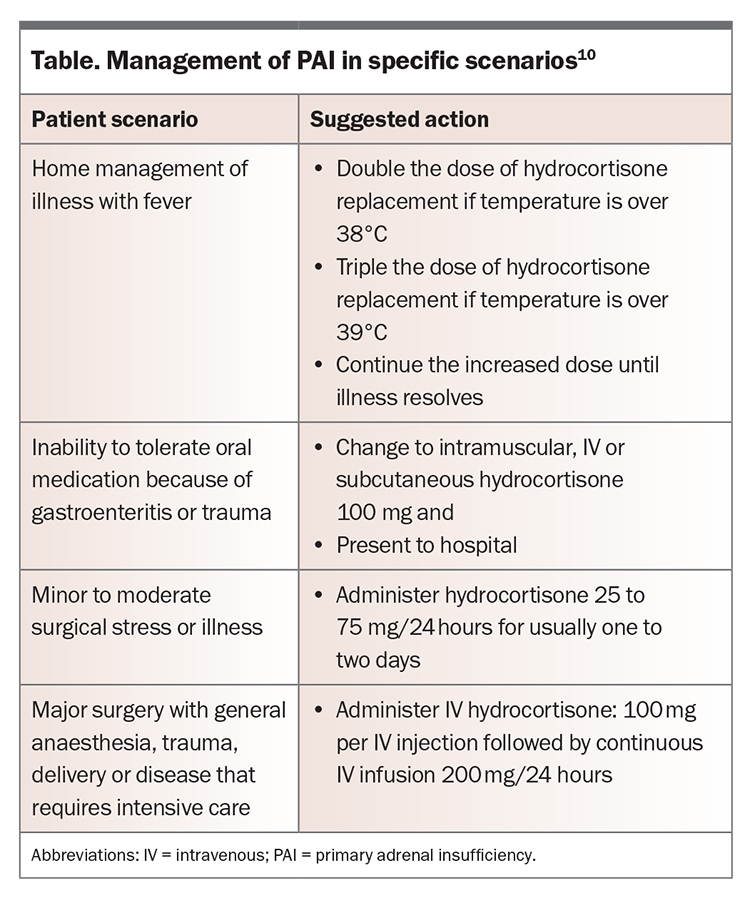
Infection is a common precipitant, with bacterial and viral infections being more common in adults and children, respectively.21,22 Gastroenteritis can reduce the absorption of oral medications. Medication nonadherence can also precipitate an adrenal crisis, as can abrupt cessation of glucocorticoid therapy by a clinician.22,23 Patients who are undergoing major surgery or have sustained a serious injury cannot mount a sufficient endogenous cortisol response to balance the pathophysiological elevation of acute-phase cytokines and require supplemental hydrocortisone (‘stress dosing’).21 Management and hydrocortisone doses to help reduce the risk of adrenal crisis in these situations are shown in the Table.
Medications and substances that induce the cytochrome P450 3A4 enzyme (CYP3A4), such as rifampicin, phenytoin, mitotane and St John’s wort, increase the metabolism of hydrocortisone and can induce an adrenal crisis in patients with undiagnosed adrenal insufficiency. Conversely, CYP3A4 inhibitors such as grapefruit juice may inhibit the metabolism of hydrocortisone, leading to increased circulating cortisol that further suppresses endogenous cortisol and increases the risk of adrenal insufficiency on withdrawal.24
In patients with untreated adrenal insufficiency and concomitant hypothyroidism, commencement of thyroxine replacement may precipitate adrenal crisis, as thyroxine increases the activity of the cortisol-inactivating enzyme 11beta-hydroxysteroid dehydrogenase.25
Treatment of adrenal crisis
Adrenal crisis is a medical emergency. The annual risk of adrenal crisis per patient is 6 to 8%, but the risk is unevenly distributed, with some patients appearing prone to events and others having no events over many years.7,26 Immediate treatment with intravenous hydrocortisone, given as a 100 mg bolus followed by 200 mg every 24 hours as a continuous infusion or frequent intravenous injection (50 mg every six hours), will produce sufficient cortisol levels, likely to exceed those seen in disease states such as severe sepsis.4 Hydrocortisone doses for children are 50 to 100 mg/m2 as a bolus followed by 50 to 100 mg/m2 over 24 hours, divided into four six-hourly doses. Subsequent hydrocortisone doses can be tailored based on clinical response. Equivalent doses of other types of parenteral steroids, such as prednisolone or methylprednisolone, may be used if hydrocortisone is not available.
When the hydrocortisone dose is greater than 50 mg daily, fludrocortisone is not required because hydrocortisone has a physiologically significant mineralocorticoid effect at this dose. Fludrocortisone is usually resumed at the pre-crisis dose after resolution of the adrenal crisis.
Intravenous normal saline should be administered as part of resuscitation of a patient with an adrenal crisis. This is typically 1000 mL of 0.9% saline over one hour in adults (children, bolus 20 mL/kg of 0.9% saline, up to 60 mL/kg in one hour if needed for shock) and then continued rates depending on patient haemodynamic status.6 This should be adjusted based on clinical assessment and other relevant conditions.7 Intravenous glucose 5% in normal saline is given in the setting of hypoglycaemia, which is more often seen in children with adrenal crisis.7
Preventing adrenal crisis
Key strategies to prevent adrenal crisis include:
- education and provision of a sick-day management plan
- supply of parenteral hydrocortisone and instructions on its use
- provision of devices such as a medical alert bracelet or necklace.
Stress dosing of glucocorticoids is essential to mitigate the risk of adrenal crisis, as this mimics the physiological response to illness. Depending on the intensity of the stressor, patients with known adrenal insufficiency need to double or triple their oral hydrocortisone dose (Table).7,10 Although temporarily increasing the dose of glucocorticoids is necessary in specific situations, such as during acute illness or stress, there is no evidence to support the routine use of supraphysiological doses for the prevention of adrenal crisis.
Patients with PAI and their family members should be well educated on the technique of intramuscular hydrocortisone injection for use when the patient is severely unwell or not tolerating oral medication because of vomiting or diarrhoea.7 Subcutaneous injection may be an alternative for those who are reluctant to use intramuscular hydrocortisone.27 In some situations, rectal prednisolone can be used, especially when there is vomiting but no diarrhoea.
Carrying a ‘steroid card’ or wearing a medical alert bracelet that records the diagnosis and recommendation for parenteral hydrocortisone are crucial strategies to ensure health attendants are aware of the diagnosis of adrenal insufficiency and need to administer hydrocortisone promptly.7
Long-term management of PAI
A collaborative care model involving the GP, endocrinologist and clinical nurse specialist via Team Care Arrangements can be important to provide ongoing care for patients with PAI and minimise the risk of adrenal crisis. GPs can formulate a GP Management Plan with patients. This may involve the GP in a holistic view of patient care and additional healthcare providers based on the specific needs of the patient, as discussed with their doctor. Generally, an endocrine nurse, accessed via an endocrine specialist, can provide detailed personalised instruction on adrenal crisis prevention (including stress dosing, hydrocortisone for injection and use of a medical alert bracelet). GPs can ensure patients are reminded of sick-day management, including the need to increase the hydrocortisone dose in specific situations (Table).10 Patients should also be supplied with a sufficient supply of hydrocortisone and fludrocortisone, accounting for possible sick days.
Specialist review, often annual, may incorporate assessment of the adrenal hormone dose over time based on experience, clinical manifestations of over- and underdosing and results of investigations such as serum electrolytes and renin levels and bone density. Bone loss has been less prevalent in the past two decades as glucocorticoid doses have generally been lower; however, low doses of short-acting glucocorticoids may increase the risk of adrenal crisis.7 In addition, screening for associated autoimmune conditions, such as autoimmune gastritis, coeliac disease and type 1 diabetes mellitus, can be arranged, and the risk of premature ovarian insufficiency considered when relevant.
Conclusion
Glucocorticoid replacement therapy with regular monitoring can restore wellbeing to a substantial proportion of patients with PAI. However, all patients with PAI are at risk of adrenal crisis. Recognition of the precipitants of adrenal crisis is essential to prevent episodes. The risk of an adrenal crisis can be mitigated by patient education on sick-day management and stress dosing, with regular follow up by a GP as well as an endocrinologist. Unfortunately, these measures cannot completely obviate the risk of adrenal crisis. ET
COMPETING INTERESTS: None.
References
1. Erischsen MM, Lovas K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160: 233-237.
2. Wallace I, Cunningham S, Lindsay J. The diagnosis and investigation of adrenal insufficiency in adults. Ann Clin Biochem 2009; 46: 351-367.
3. Laureti S, Vecchi L, Santeusanio F, Falorni A. Is the prevalence of Addison’s disease underestimated? J Clin Endocrinol Metab 1999; 87: 1762.
4. Meyer G, Neumann K, Badenhoop K, Linder R. Increasing prevalence of Addison’s disease in German females: health insurance data 2008-2012. Eur J Endocrinol 2014; 170: 367-373.
5. Addison T. On the constitutional and local effects of diseases of the supra-renal capsules. London: Samuel Highley; 1855.
6. Bornstein S, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 364-389.
7. Rushworth R, Torpy D, Falhammar H. Adrenal crisis. N Engl J Med 2019; 381: 852-861.
8. Abdel-Malek ZA, Knittel J, Kadekaro AL, Swope VB, Starner R. The melanocortin 1 receptor and the UV response of human melanocytes-a shift in paradigm. Photochem Photobiol 2008; 84: 501-508.
9. Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci 2010; 339: 525-531.
10. Allolio B. Extensive expertise in endocrinology: adrenal crisis. Eur J Endocrinology 2015; 172: R115-R124.
11. El-Farhan N, Pickett A, Ducroq D, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol (Oxf) 2013; 78: 673-680.
12. Yo WS, Toh LM, Brown SJ, Howe WD, Henley DE, Lim EM. How good is a morning cortisol in predicting an adequate response to intramuscular Synacthen stimulation? Clin Endocrinol (Oxf) 2014; 81: 19-24.
13. Ebeling PR. Death after failure to diagnose Addison disease. Aust Fam Physician 2008; 37: 6.
14. Selmer C, Olesen JB, Hansen ML, et al. Subclinical and over thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab 2014; 99: 2372-2382.
15. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short Synacthen test. Clin Endocrinol (Oxf) 2013; 79: 14-19.
16. Werumeus Bunning J, Touw DJ, et al. Pharmacokinetics of oral hydrocortisone – results and implications from a randomised controlled trial. Metabolism 2017; 71: 7-16.
17. Oelkers W, L’Age M. Control of mineralocorticoid substitution in Addison’s disease by plasma renin measurement. Wiener Klinische Wochenschrift 1976; 54: 607-612.
18. Oelkers W, Diederich S, Bahr, V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinology Metabolism 1992; 75: 259-264.
19. Inder WJ, Meyer C, Hunt PJ. Management of hypertension and heart failure in patients with Addison’s disease. Clin Endocrinol 2015; 82: 789-792.
20. Hafner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab 2015; 100: 407-416.
21. Smans LC, Van der Valk ES, Hermus AR, Zelissen PM. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2016; 84: 17-22.
22. Rushworth RL, Torpy D. A descriptive study of adrenal crises in adults with adrenal insufficiency: increased risk with age and in those with bacterial infections. BMC Endocr Disord 2014; 14: 79.
23. Burger-Stritt S, Kardonski P, Pulzer A, Meyer G, Quinkler M, Hahner S. Management of adrenal emergencies in educated patients with adrenal insufficiency – a prospective study. Clinc Endocrinol (Oxf) 2018; 89: 22-29.
24. Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med 2009; 360: 2328-2339.
25. Lewandowski KC, Marcinkowska M, Skowrońska-Jóźwiak E, Makarewicz J, Lewiński A. New onset Graves’ disease as a cause of an adrenal crisis in an individual with panhypopituitarism: brief report. Thyroid Res 2008; 1: 7.
26. Klauer KM. Adrenal insufficiency and adrenal crisis: differential diagnoses and workup, 2009. Available online at: http://emedicine.medscape.com/article/765753 – diagnosis (accessed October 2010).
27. Pulzer A, Meyer G, Quinkler M, Hahner S. Management of adrenal emergencies in educated patients with adrenal insufficiency – a prospective study. Clin Endocrinol (Oxf) 2018; 89: 22-29.