Short stature in children – when to consider growth hormone deficiency

Children generally follow a growth trajectory that is consistent with their genetic potential. Most children with a height less than the third percentile have familial short stature or constitutional delay. Growth hormone (GH) deficiency is a rare cause of short stature. Assessing a child with short stature requires a comprehensive history, examination, investigations and monitoring. GH therapy is available through the PBS for GH deficiency and a number of other conditions associated with short stature. Specialist referral should be considered for a child with severe short stature or slow growth with no medical cause.
- Short stature is defined as a height more than two standard deviations below the mean.
- A child with short stature should be assessed for height, weight and general health, including calculating a mid-parental height and monitoring the child’s height to calculate a growth velocity.
- Growth hormone deficiency is characterised by slow growth velocity, delayed bone age and low insulin growth factor-1 levels. It is a rare cause of short stature.
- Referral to a paediatrician or paediatric endocrinologist should be considered if a child’s height is below the first percentile, the growth velocity is below the 25th percentile, or if there is discrepancy between the child’s height and the mid-parental height.
- The PBS subsidises GH treatment for children with GH deficiency and a number of other conditions associated with short stature.
Growth in children and adolescents
Growth in the first two years in children is dependent on nutritional, hormonal and environmental factors. From the age of about 3 years, children generally follow a growth trajectory consistent with their genetic potential. Some healthy children will cross percentiles between infancy and childhood as they establish their growth trajectory. There is often a fall in growth velocity just before a child enters puberty.
Short stature is defined as a height more than two standard deviations (SD) below the population mean (<–2 SD). Thus, about 2.5% of the population will be defined as having short stature. Being shorter than average is very rarely a clinical problem. Most short children are small due to their genetic potential. Very rarely, some children will be short due to a genetic condition or a chronic disease.
Growth hormone (GH) deficiency is a very rare cause of short stature. The prevalence of GH deficiency is about two to three per 10,000 children. Children with congenital GH deficiency are generally short. However, those with acquired GH deficiency may not have short stature, but will demonstrate a decrease in growth velocity.
Assessing growth
Measurement of height and weight is an essential feature of the physical examination of children. In children aged under 2 years, length is preferred to height.1 In older children who can stand, height should be measured using a calibrated stadiometer. Taking multiple measurements over time, ideally every three months, is recommended to assess height velocity (Figure). A single height measurement does not evaluate growth.
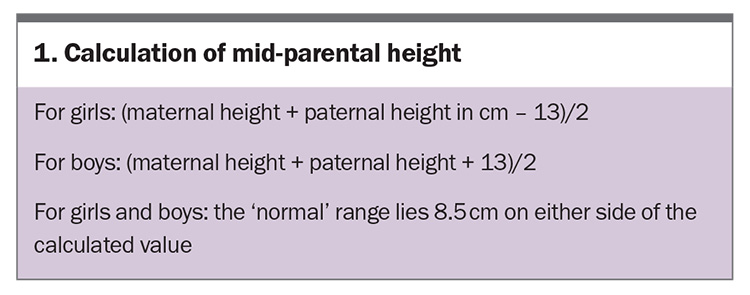
The Australia and New Zealand Society for Paediatric Endocrinology and Diabetes (ANZSPED) recommends the WHO growth charts for children younger than 2 years of age and the Centers for Disease control and Prevention (CDC) growth reference charts for older children.2 The growth charts in practice software are sufficient for most children; however, if there is a concern about a child’s growth velocity or if their height is below the third percentile, the use of validated growth charts is recommended. Significant variability in height potential exists among different ethnic groups.3 A measurement of mid-parental height (Box 1) is a useful predictor of a child’s adult height and takes into account their genetic potential (Box 2).
In a child with short stature, an assessment of their growth requires taking a comprehensive history, performing a physical examination and reviewing their growth trajectory.4 Important features to elicit include whether the child was conceived via in vitro fertilisation, perinatal events, birth parameters (weight, height and head circumference) and hypoglycaemia and jaundice in the neonatal period. Comparing the child’s height with the mid-parental height (Box 1) helps to determine if the child’s height is consistent with the genetic potential. The expected final height (based on the bone age) of a child should fall within 8.5 cm of the mid-parental height. It is also important to ask about the child’s nutrition and medication use (especially inhaled and oral corticosteroid use). The physical examination should include measurement of height, weight and body proportions, as well as assessing pubertal stage, visual fields and systemic health.5-7
Investigations to consider for growth assessment include a bone age study and screening blood tests. A bone age study is an x-ray of the hand that is used to assess the epiphyses of the carpals, metacarpals, radius and ulnar.8 Screening blood tests include:
- tests to exclude chronic disease (electrolyte levels, liver function tests, calcium, magnesium and phosphate levels, tissue transglutaminase antibodies, full blood count, thyroid function tests and iron studies)
- karyotype screening for girls with height below the first percentile to exclude Turner syndrome, even if no other clinical features are present
- karyotype screening or a microarray for a child with dysmorphic or syndromal features
- screening for GH deficiency – measuring a random GH level is not a helpful to test for GH deficiency; insulin growth factor (IGF)-1 is a better marker of GH sufficiency. A low IGF-1 level can have many causes but a normal IGF-1 level generally indicates GH sufficiency. IGF binding protein-3 can be helpful in children younger than 3 years of age or who are underweight
- measurement of luteinising hormone, follicle-stimulating hormone, oestradiol or testosterone levels could be considered in adolescents with delayed onset of puberty.
When to refer
A child should be referred for specialist assessment if their height is well below the first percentile or is predicted to be more than 8.5 cm below that of their mid-parental height. A child with a slow growth velocity (below the 25th percentile for bone age) or who crosses percentiles should be evaluated closely and referred if there are no nutritional or medical reasons for their slow growth as they may have GH deficiency. A simple algorithm for assessing a child with suspected short stature is presented in the Flowchart.
Causes of short stature (that are not GH deficiency)
There are many syndromes associated with short stature, the most common being Turner syndrome and Down syndrome. Chronic diseases, especially those associated with poor nutrition, inflammation or the use of high doses of glucocorticoids can impair growth. Treating the underlying condition and avoiding the use of steroids is essential in these disorders.
There is increasing recognition of a number of genetic disorders affecting the growth plate or causing mild forms of skeletal dysplasia that can be associated with short stature. Examples include abnormalities in the CYP26C1, SHOX, ACAN, NPR2 or IHH genes.9
In Australia, genetic testing is best arranged through the local public genetics service. Referral should be considered for severe short stature (height <–3 SD) and short stature associated with other syndromal features. Genetic testing could also be considered for a child born small for gestational age who does not catch up in height.10 Having a genetic diagnosis for short stature can help with understanding its pathogenesis and guide treatment decisions.
Physiological variation – familial short stature and constitutional delay
It can be challenging to differentiate between constitutional delay and GH deficiency. Constitutional delay is variant of normal growth and is characterised by a delayed bone age. The estimated final height based on the delayed bone age is consistent with the mid-parental height. IGF-1 levels corrected for pubertal stage will be normal. There may be delayed onset of puberty. If GH stimulation tests are performed in a child with suspected constitutional delay, sex steroid priming is essential.
A child with familial short stature will have a predicted adult height within 8.5 cm of the mid-parental height, based on their bone age. In both of these conditions, growth velocity is normal.
Causes of slow growth
Poor nutrition
Infants, children with chronic disease and children or adolescents with eating disorders can experience poor growth due to poor nutrition. The key feature in these situations is that the decrease in weight percentile occurs before the decrease in height percentile.
Short stature and slow growth
Short stature and slow growth is sometimes called ‘idiopathic short stature’ and refers to very short children (height below the first percentile) who are growing slowly with no defined pathological cause. These children may be eligible for GH therapy based on their auxological criteria. However, they are not GH deficient, based on normal IGF-1 levels or normal GH stimulation tests.
Growth hormone deficiency
GH is synthesised and secreted in a pulsatile manner by the anterior pituitary somatotrophs throughout the lifespan. During puberty, a threefold increase in pulsatile GH secretion occurs that peaks at mid-puberty in both boys and girls. GH secretion declines with age. IGF-1 mediates the effects of GH.
GH deficiency can be isolated or can occur in the context of multiple pituitary hormone deficiency. Where there is multiple pituitary hormone deficiency, patients may also require treatment with thyroxine, hydrocortisone and, in adolescents, oestrogen or testosterone.
Diagnosing GH deficiency
Children with GH deficiency have a slow growth velocity and delayed bone age. They tend to have a higher weight than height percentile. IGF-1 levels are used to screen for GH deficiency. Most laboratories report IGF-1 normal ranges for age; however, using the IGF-1 range for the child’s bone age or pubertal stage is more sensitive and specific.11-13 IGF-1 is not a reliable screening test in children younger than 3 years of age or children with undernutrition, delayed puberty, hypothyroidism, cancer, renal failure or brain tumours. IGF binding protein-3 (IGF-BP3) is more sensitive than IGF-1 and is the recommended screening molecule for these children.14
GH stimulation tests are generally considered for children with a low growth velocity, low IGF-1 or IGF-BP3 levels, or if there is discordance between growth rates and blood tests.15 They can be helpful to aid the diagnosis, but need to be interpreted in the right clinical context. GH stimulation tests require a half day admission to hospital. They are generally performed at one of the tertiary children’s hospitals. Testing involves giving a pharmacological stimulus to provoke GH secretion. In Australia, GH deficiency is defined as GH peak of response to two stimuli of below 3.3 pmol/L or below 10 mIU/L.
Growth hormone therapy
Prescribing and funding growth hormone therapy
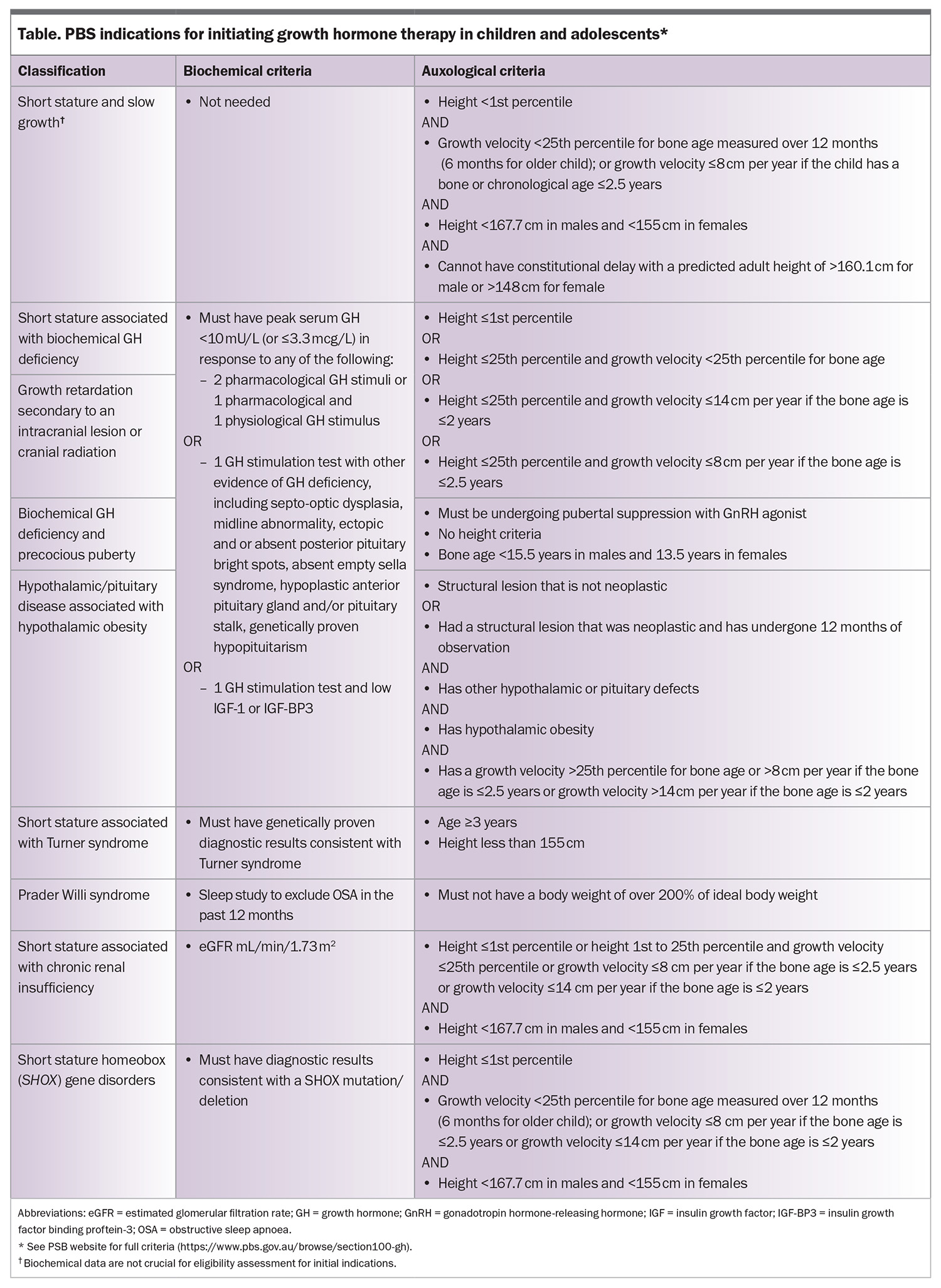
The prescribing of GH is done by a paediatric endocrinologist or paediatrician. It is funded through the PBS via the GH program (https://www.pbs.gov.au/browse/section100-gh) for a number of conditions (Table). Patients are given an authority prescription for three months with one repeat. An endocrine nurse specialist or representative from the GH company teaches the patient or parent how to administer the growth hormone.
GH is a controlled substance.16 A letter stating the medical need for GH is required for patients who are travelling.
Growth hormone formulations
Somatropin
Somatropin is manufactured using recombinant DNA technologies and is available in Australia under a number of trade names. It is given as a nightly injection. The dosing regimens are either six or seven days, based on the most efficient way to deliver the weekly dose. The recommended dose in Australia is between 4.5 and 7.5 mg/m2/week and is titrated to achieve optimal growth and normal IGF-1 level. Children are unlikely to have any acute problems from missing a dose, with the exception of infants with multiple pituitary hormone deficiency who may be prone to hypoglycaemia.
Long-acting GH preparations
Although children are motivated to take their GH dose to optimise their growth, like many medications for chronic disease, compliance can be an issue. This has led to the development of multiple long-acting GH treatments, including somatrogon and somapacitan. The dosing of the long-acting preparations is a fixed dose per kg. The dose is titrated by keeping the IGF-1 level within the normal range. A blood test to measure IGF-1 level should be done four days after the injection is administered.17-20
Potential side effects
GH may occasionally cause unwanted side effects, such as skin reactions at the injection sites and, less often, headaches, swelling of the arms and legs or limping. In children who develop headaches after starting GH therapy, benign intracranial hypertension should be considered. Treatment should be stopped and recommenced at a lower dose. In patients who limp or have hip pain after starting GH therapy, an x-ray should be performed to evaluate for slipped femoral capital epiphysis. Children with bone disorders such as scoliosis need to be closely monitored as rapid growth can aggravate these problems. GH can rarely also aggravate insulin resistance and type 2 diabetes.
Transition to adult care
GH is now approved for use in adults with GH deficiency. Children with biochemically proven GH deficiency associated with a congenital, structural or genetic condition are eligible for PBS-funded GH therapy after skeletal maturation and in adulthood. However, children with isolated GH deficiency require retesting for PBS-funded GH therapy.
The dose of GH required for adults is lower than that for children. An adult dose of GH is used when the bone age is over 13.5 years in girls and 15.5 years in boys.
Conclusion
GPs have an important role in monitoring the growth of children and adolescents, and referring them for assessment if their growth falls beyond the expected pattern for their genetic potential. Children may be treated with GH for GH deficiency, or to treat other causes of short stature. GH is prescribed by a paediatrician, paediatric endocrinologist or adult endocrinologist. GPs are encouraged to keep up to date on the indications for GH treatment, potential side effects and other common issues that might arise while a patient is taking GH (when travelling, missed doses, etc). ET
COMPETING INTERESTS: Dr Stone has received payment for a lecture from Pfizer. Dr Nyunt has received an investigator-initiated research grant from Pfizer.
References
1. Ribeiro DS, Sasinski J, Hackett H, Manalo C, Choi J, Miller PS. Comparison of infant length measurements using tape measure versus length board. Adv Neonatal Care 2023; 23: 435-441.
2. Australia and New Zealand Society for Paediatric Endocrinology and Diabetes (ANZSPED). Growth and Growth Charts. ANZSPED; NSW, 2020. Available online at: https://anzsped.org/clinical-resources-links/growth-growth-charts/ (accessed May 2024).
3. Mittal M, Gupta P, Kalra S, Bantwal G, Garg MK. Short stature: understanding the stature of ethnicity in height determination. Indian J Endocrinol Metab 2021; 25: 381-388.
4. Wit JM, Kamp GA, Oostdijk W, Dutch Working Group on Triage and Diagnosis ofGrowth Disorders in Children. Towards a rational and efficient diagnostic approach in children referred for growth failure to the general paediatrician. Horm Res Paediatr 2019; 91: 223-240.
5. Engstrom FM, Roche AF, Mukherjee D. Differences between arm span and stature in white children. J Adolesc Health Care 1981; 2: 19-22.
6. Gerver WJM, Gkourogianni A, Dauber A, Nilsson O, Wit JM. Arm span and its relation to height in a 2- to 17-year-old reference population and heterozygous carriers of ACAN variants. Horm Res Paediatr 2020; 93: 164-172.
7. Hertel NT, Scheike T, Juul A, et al. Body proportions of Danish children. Curves for sitting height ratio, subischial length and arm span. Ugeskr Laeger 1995; 157: 6876-6881.
8. Satoh M, Hasegawa Y. Factors affecting prepubertal and pubertal bone age progression. Front Endocrinol (Lausanne) 2022; 13: 967711.
9. Wit JM DA, Bin-Abbas B, Al Mutair A, Koledova E, Savage MO. Achieving optimal short- and long-term responses to paediatric growth hormone therapy. Journal Clin Res Paediatr Endocrinol 2019; 11: 329-340.
10. Collett-Solberg PF, Ambler G, Backeljauw PF, et al. Diagnosis, genetics, and therapy of short stature in children: a growth hormone research society international perspective Horm Res Paed 2019; 92: 1-14.
11. Cole TJ, Ahmed ML, Preece MA, Hindmarsh P, Dunger DB. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol (Oxf) 2015; 82: 862-869.
12. Inoue-Lima TH, Vasques GA, Scalco RC, et al. IGF-1 assessed by pubertal stage has the best positive predictive power for GH deficiency diagnosis in pre-pubertal children. J Pediatr Endocrinol Metab 2019; 32: 173-179.
13. Clemmons DR. Consensus statement on the standardisation and evaluation of GH and IGF-1 assays. Clin Chem 2011; 57: 555-559.
14. Wit JM, Bidlingmaier M, de Bruin C, Oostdijk W. A proposal for the interpretation of serum IFG-1 concentration as part of laboratory screening in children with growth failure. J Clin Res Pediatr Endocrinol 2020; 12: 130-139.
15. Yau M, Rapaport R. Growth hormone stimulation testing: to test or not to test? that is one of the questions. Front Endocrinol (Lausanne) 2022; 13: 902364.
16. Australian Government Department of Health and Aged Care. Office of Drug Control. List of controlled substances. Available online at: https://www.odc.gov.au/controlled-substances/list (acceded May 2024).
17. Zadik Z, Zelinska N, Iotova V, et al. An open-label extension of a phase 2 dose-finding study of once-weekly somatrogon vs. once-daily genotropin in children with short stature due to growth hormone deficiency: results following 5 years of treatment. J Pediatr Endocrinol Metab 2023; 36: 261-269.
18. Savendahl L, Battelino T, Hojby Rasmussen M, et al. Effective GH replacement with once-weekly somapacitan vs daily GH in children with GHD: 3-year results from REAL 3. J Clin Endocrinol Metab 2022; 107: 1357-1367.
19. Savendahl L, Battelino T, Hojby Rasmussen M, et al. Weekly somapacitan in GH deficiency: 4-year efficacy, safety, and treatment/disease burden results from REAL 3. J Clin Endocrinol Metab 2023; 108: 2569-2578.
20. Savendahl L, Battelino T, Brod M, et al. Once-weekly somapacitan vs daily GH in children with GH deficiency: results from a randomized phase 2 trial. J Clin Endocrinol Metab 2020; 105: e1847e18-61.