NAFLD in people with type 2 diabetes – improving health outcomes
Nonalcoholic fatty liver disease (NAFLD) is highly prevalent in people with type 2 diabetes and is often encountered as an incidental finding in general practice. NAFLD risk stratification and management can be incorporated into the diabetes annual cycle of care, and requires consideration of underlying metabolic risk factors, and surveillance for the development of advanced liver disease.
- Nonalcoholic fatty liver disease (NAFLD) is increasingly prevalent and is a leading cause of chronic liver disease in Australia.
- NAFLD is common in people with type 2 diabetes and the combination is associated with an increased risk of developing advanced fibrosis and liver-related complications.
- Assessment of liver disease severity is required in patients with NAFLD to detect those at risk of advanced fibrosis who may benefit from referral to specialist hepatology services and ongoing surveillance for liver-related complications.
- GPs remain at the forefront of NAFLD diagnosis and management due to their central role in the care of people with cardiometabolic disease and associated risk factors.
- Although targeted pharmacotherapy for NAFLD is not yet available, the cornerstone of treatment continues to be lifestyle measures and medical management of associated cardiometabolic risk factors.
About 70% of people with type 2 diabetes have nonalcoholic fatty liver disease (NAFLD), the liver component of a group of conditions associated with metabolic dysfunction.1 First characterised in 1980, the prevalence of NAFLD has risen globally in conjunction with rates of obesity.2,3 NAFLD is the most common cause of chronic liver disease in Australia, with an estimated prevalence of 20 to 30% in the general population, accounting for 90% of the liver disease burden.4,5 By 2030, the prevalence of NAFLD is estimated to increase by 25%.6
Although most people with NAFLD do not develop clinically significant liver disease, 2 to 3% develop advanced fibrosis and are at risk of developing complications of cirrhosis, including hepatocellular carcinoma (HCC), over a period of 10 to 20 years.7 Type 2 diabetes is an important risk factor for the development of liver fibrosis and liver disease complications. A key issue for GPs is the ability to detect which patients are at greatest risk of developing liver complications among the very large number of affected individuals. This at-risk subgroup may benefit from linkage with specialist hepatology services and surveillance for liver decompensation and HCC.
What is NAFLD and why does it matter in people with type 2 diabetes?
NAFLD is defined by the presence of steatosis in more than 5% of hepatocytes in association with metabolic risk factors (particularly obesity and type 2 diabetes) and in the absence of excessive alcohol use (≥30 g/day for men and ≥20 g/day for women) or other chronic liver diseases. A new term metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed to highlight the role of cardiometabolic risk factors and does not require the exclusion of other chronic liver diseases.8
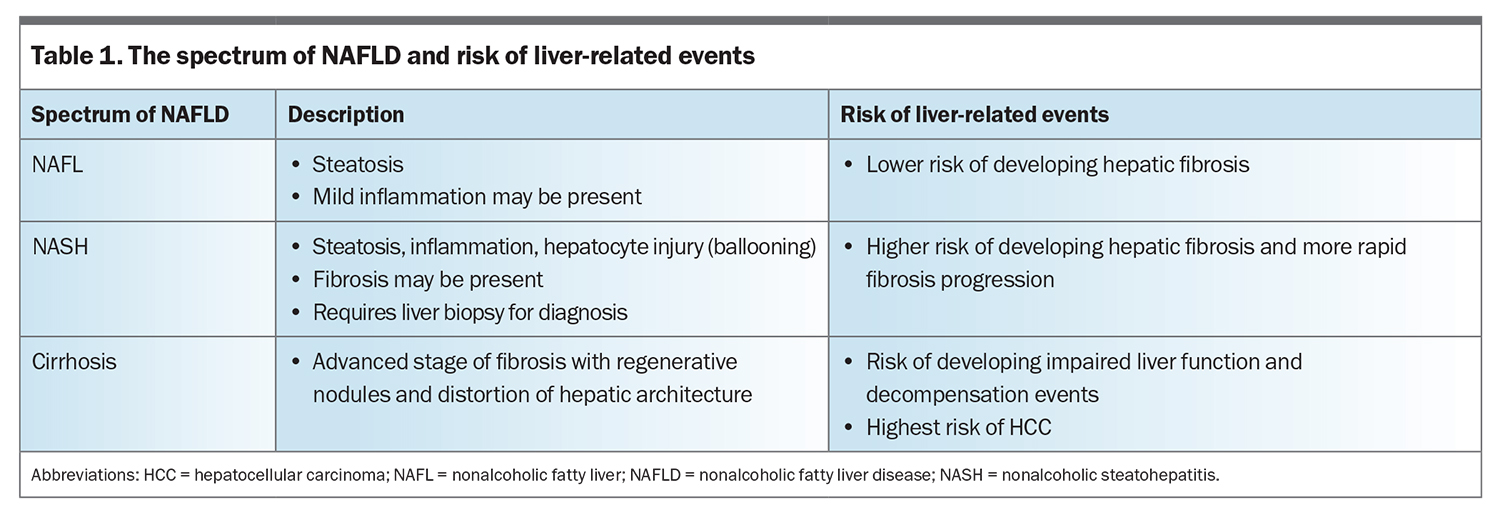
NAFLD can be considered as a spectrum, ranging from nonalcoholic fatty liver (NAFL) without significant inflammation, to nonalcoholic steatohepatitis (NASH), in which hepatic steatosis is associated with liver cell injury (hepatocyte ballooning) and inflammation, with or without fibrosis (Table 1).9 Although fibrosis may develop in both NAFL and NASH, fibrosis progression occurs at a more rapid rate in patients with NASH.10 Cirrhosis refers to an advanced stage of liver fibrosis, with formation of regenerative nodules and distortion of the hepatic architecture, which may lead to impaired hepatic function and HCC.
Type 2 diabetes (irrespective of body weight) is an important driver of NAFLD progression, and is associated with a greater than twofold increase in the risk of developing advanced fibrosis, cirrhosis-related complications and liver disease mortality.11 People with obesity and multiple metabolic comorbidities are also at risk of significant liver disease.
Is screening for NAFLD in people with type 2 diabetes worthwhile?
Some guidelines recommend screening for NAFLD in high-risk populations (e.g. people with type 2 diabetes, obesity or metabolic syndrome). The American Association of Clinical Endocrinology recommends screening people with type 2 diabetes for NAFLD to identify people at risk of liver disease progression and provide them with early intervention and referral (if needed) to a hepatologist (or gastroenterologist with an interest in liver disease).12 Reasons for not screening include: limitations with the noninvasive assessment of NAFLD; no approved drugs for the treatment of NAFLD; and lack of knowledge about whether screening is cost effective and improves patient outcomes.
All patients diagnosed with NAFLD require an assessment of the severity of liver disease and their risk of cardiovascular disease and comorbid conditions. The most important predictor of mortality in NAFLD is the extent of liver fibrosis. In particular, the presence of advanced fibrosis or cirrhosis is associated with increased risk of overall and liver-related mortality.13 Cirrhosis is also the strongest risk factor for the development of HCC. Early detection of advanced fibrosis is important to allow at-risk patients to be referred for specialist hepatology care and to undergo surveillance for liver cancer before they develop liver-related complications.
How is NAFLD diagnosed?
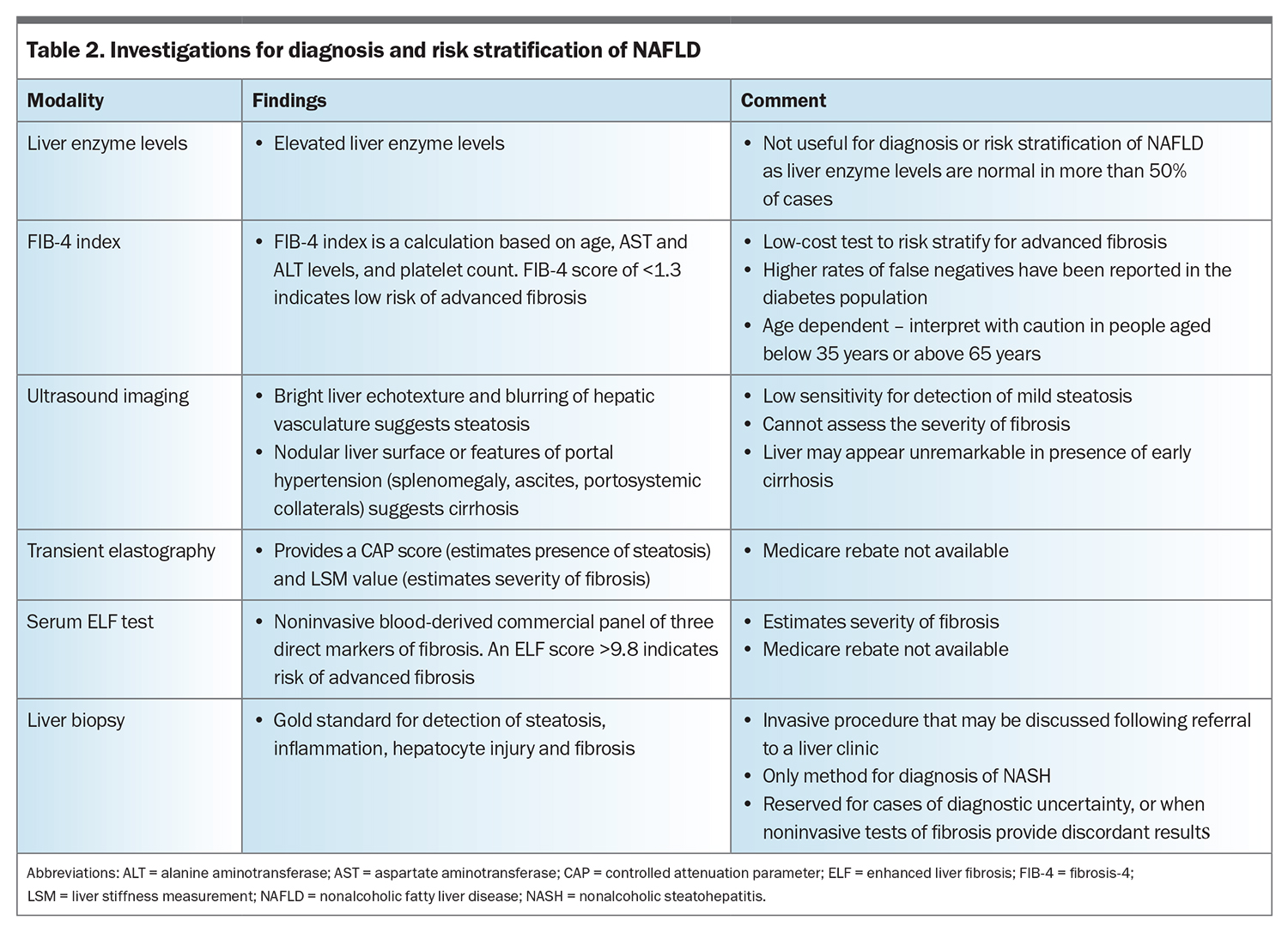
The diagnosis of NAFLD requires demonstration of hepatic steatosis (most often by abdominal ultrasonography, Table 2) and the exclusion of secondary causes of steatosis such as excess alcohol consumption. A liver ultrasound is recommended to assess suspected fatty liver; however, when steatosis is mild (<30%), this test has low sensitivity. Controlled attenuation parameter (CAP), an ultrasound-based technique, can be used to assess steatosis, and can be performed at the same time as testing for advanced fibrosis using transient elastography. As the chance of having steatosis is very high for people with type 2 diabetes (about 70%), a liver ultrasound is not required before assessment of liver disease severity.
Liver function tests are not useful for a diagnosis of NAFLD, as the results are normal in more than 50% of cases. All patients with abnormal liver enzyme levels need to have other common causes of liver disease excluded, by screening for unhealthy alcohol use, reviewing prescribed and nonprescribed medications and testing for hepatitis B and C infection.
How should severity of liver disease be assessed?
First-step fibrosis test
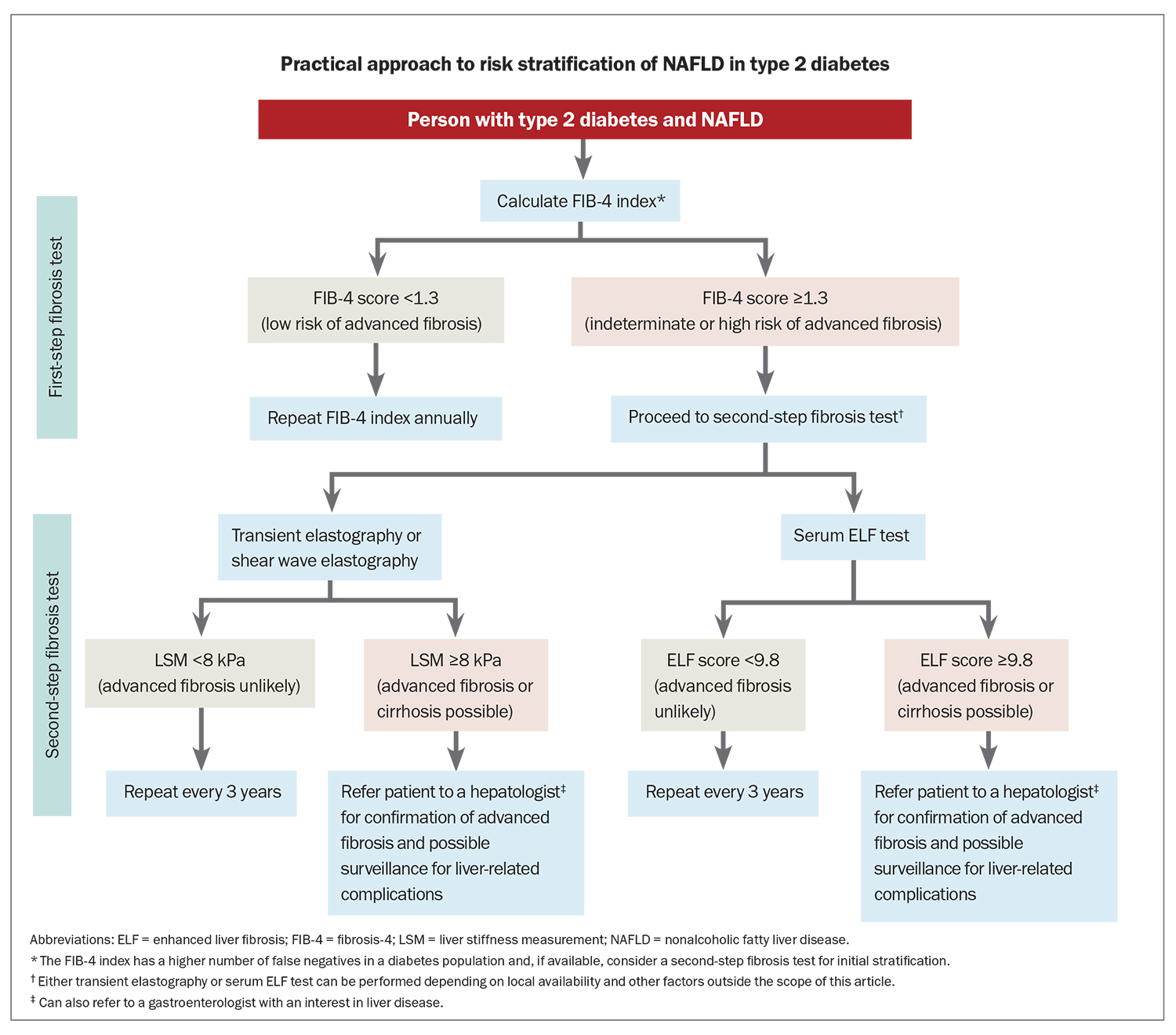
Clinical judgement and measurement of liver enzyme levels cannot determine the severity of NAFLD; therefore, noninvasive biomarkers are used to calculate the risk of advanced fibrosis (Table 1 and the Flowchart). The Fibrosis-4 (FIB-4) index is recommended as the first step to identify people at low risk of advanced fibrosis (FIB-4 score <1.3) who can be managed in primary care.12,14,15 It is a low-cost algorithm available online (www.mdcalc.com/calc/2200/fibrosis-4-fib-4-index-liver-fibrosis) that uses a patient’s age and common laboratory results (aspartate aminotransferase and alanine aminotransferase levels and platelet count) to calculate a score. The score has a high negative predictive value to exclude advanced liver fibrosis, although may be less accurate in people with type 2 diabetes.16 People without advanced fibrosis at initial assessment need repeat testing over time to identify progression of liver disease. The FIB-4 index can be measured yearly as part of the diabetes annual cycle of care.
Second-step fibrosis tests
People with a FIB-4 score greater than low risk (≥1.3) need further assessment with a locally available second-step fibrosis test, such as ultrasound-based elastography or the enhanced liver fibrosis (ELF) test. Transient elastography with FibroScan can be performed as a rapid point-of-care test and a liver stiffness measurement (LSM) of less than 8.0 kPa has high sensitivity (93%) to exclude advanced fibrosis. LSM values of more than 12 kPa can be used to rule in advanced fibrosis or cirrhosis.17 Shear wave elastography (available at many private radiology centres) has a similar performance to transient elastography, although has not been as well validated.
The ELF test is a noninvasive blood-derived panel of three direct markers of fibrosis, and is one of the most validated patented serum fibrosis tests. An ELF score of 9.8 or more indicates advanced fibrosis and a score of 11.3 or more indicates cirrhosis.18 The ELF test can be requested through Sonic Healthcare Australia Pathology (using the term ‘serum liver fibrosis markers’).
People with type 2 diabetes and an LSM value of less than 8 kPa or ELF test of less than 9.8 remain at risk of developing advanced fibrosis and require repeat testing every three years. A Medicare rebate is not available for these second-step fibrosis tests, which is a limiting factor for their use in the community at present.
Why are noninvasive tests used in a stepwise manner to assess the severity of liver disease?
None of the currently available noninvasive tests are completely accurate, and all have certain benefits or disadvantages. In general practice, where the prevalence of advanced fibrosis is low, noninvasive tests are usually better at ruling out advanced fibrosis using low-risk scores. As the FIB-4 index has a low positive predictive value for advanced fibrosis, further tests are required for indeterminate or high-risk scores.
In people with type 2 diabetes, in whom there is a moderate prevalence of advanced fibrosis, the FIB-4 index has a higher number of false negatives. Therefore, if available, ultrasound-based elastography or the serum ELF test may be preferable as the initial step to assess the risk of advanced fibrosis in people with type 2 diabetes.
Who should be referred for further assessment of liver disease?
People with noninvasive tests suggesting an increased risk of advanced fibrosis or cirrhosis (transient elastography ≥8 kPa, ELF score of ≥9.8, or FIB-4 score of ≥1.3 with no second-step test available in the community) require specialist referral for further assessment of liver disease. If cirrhosis is confirmed, these patients may be offered surveillance for liver cancer, as cirrhosis is the strongest risk factor for the development of HCC.
If there is evidence of cirrhosis on liver imaging (nodular liver surface or features of portal hypertension such as splenomegaly, varices, ascites) or biochemical abnormalities suggesting advanced fibrosis (low platelet count or low serum albumin level), referral of the patient to a liver clinic is advised.
How is NAFLD managed in primary care?
Most people with type 2 diabetes and NAFLD do not require referral to specialist hepatology services and those with a low risk of advanced fibrosis can be managed in primary care. At present, there are no TGA-approved drugs for the treatment of NAFLD. Due to shared cardiometabolic risk factors, the leading cause of death in patients with NAFLD is cardiovascular disease, followed by extrahepatic malignancy. Therefore, the management of NAFLD needs to take a holistic approach with the goal of reducing the risk of cardiovascular disease as well as steatosis and liver injury.
Screening for and early treatment of obesity, dyslipidaemia and hypertension are important to reduce the risk of cardiovascular events and diabetes complications. Lifestyle changes to promote weight loss, including reduced caloric intake and increased physical activity, remain the standard of care. A healthier eating pattern such as the Mediterranean diet (low in carbohydrates and saturated fat, higher in monosaturated fat) is recommended. Weight loss of more than 5 to 7% of total body weight reduces steatosis and NASH and improves associated cardiometabolic comorbidities. A greater weight loss (>10% of total body weight) can also improve liver fibrosis. Physical activity, such as 150 minutes of brisk walking per week, remains beneficial, even in the absence of weight loss, resulting in a reduction of steatosis.19,20
Obesity pharmacotherapy can be used if the required weight loss is not achieved with lifestyle changes alone. The glucagon-like peptide-1 receptor agonist, semaglutide, is the most effective medication to date, achieving substantial weight loss (>10% total body weight), as well as treating hyperglycaemia and improving cardiometabolic risk.21 There may be particular benefit to the use of this medication in people with diabetes with concurrent obesity. Use of glucagon-like peptide-1 receptor agonists can also improve steatosis and NASH, and are currently in clinical trials for the treatment of NAFLD.22 Another drug class, sodium-glucose cotransporter-2 inhibitors, can be used to reduce the risk of major cardiovascular events and progression of chronic kidney disease in people with type 2 diabetes and NAFLD.23
In patients with severe obesity, bariatric surgery can lead to considerable sustained weight loss with improvement in NASH and liver fibrosis, along with metabolic comorbidities.24 Bariatric surgery is not recommended in patients with portal hypertension because of increased surgical risk and postoperative complications, including decompensated liver disease. Weight loss in patients with cirrhosis may lead to sarcopenia; therefore, adequate protein intake and exercise are important to maintain muscle mass.
Other metabolic comorbidities should be treated according to current guidelines. In particular, statins are safe for use in people with type 2 diabetes and NAFLD and remain a first-line therapy in treating elevated low-density lipoprotein cholesterol levels and reducing cardiovascular events.25
How is NAFLD followed up in primary care?
People with type 2 diabetes and NAFLD assessed as being at low risk for advanced fibrosis (FIB-4 score of <1.3, LSM value of <8.0 kPa or ELF score of <9.8) remain at risk of developing advanced fibrosis over time and require repeat testing. The FIB-4 index can be measured each year during the diabetes annual cycle of care, with second-step tests (ultrasound elastography or ELF test, if available), repeated every three years. A consistent increase in the value of these noninvasive tests may suggest progressive liver disease requiring further assessment, although longitudinal changes in FIB-4 score and risk of HCC and cirrhosis are still being evaluated.26
Conclusion
In people with type 2 diabetes, NAFLD requires specific consideration because of the higher risk of liver disease progression and liver-related complications. Assessment of liver disease severity is required to detect patients at risk of advanced fibrosis who may benefit from referral to specialist hepatology services and ongoing surveillance for liver-related complications. Novel therapeutics (e.g. glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors) are now available for the management of diabetes and are increasingly used for the treatment of obesity and reduction of cardiovascular events. The efficacy of these agents in people with NAFLD remains under investigation. The cornerstone of NAFLD treatment continues to be lifestyle measures and medical management of associated cardiometabolic risk factors including type 2 diabetes. ET
COMPETING INTERESTS: Dr Sarraf: None. Professor Powell has served as a consultant or advisory board member for CSL Behring and Novo Nordisk and has received an unrestricted educational grant from Siemens Healthineers.
References
1. Lomonaco R, Leiva EG, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2020; 44: 399-406.
2. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatol 2020; 72: 1605-1616.
3. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11-20.
4. Sarin SK, Kumar M, Eslam M, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2020; 5: 167-228.
5. Mahady SE, Adams LA. Burden of non-alcoholic fatty liver disease in Australia. J Gastroenterol Hepatol 201; 33 Suppl 1: 1-11.
6. Adams LA, Roberts SK, Strasser SI, et al. Nonalcoholic fatty liver disease burden: Australia, 2019-2030. J Gastroenterol Hepatol 2020; 35: 1628-1635.
7. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149: 389-397.
8. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020; 73: 202-209.
9. Kleiner DE, Brunt EM, van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol 2005; 41: 1313-1321.
10. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643-654.
11. Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med 2020; 17: e1003100.
12. Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022; 28: 528-562.
13. Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020; 158: 1611-1625.
14. Powell EE, Wong VWS, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021; 397: 2212-2224.
15. Wong VWS, Zelber-Sagi S, Cusi K, et al. Management of NAFLD in primary care settings. Liver Int 2022; 42: 2377-2389.
16. Boursier J, Hagström H, Ekstedt M, et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J Hepatol 2022; 76: 1013-1020.
17. Papatheodoridi M, Hiriart JB, Lupsor-Platon M, et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol 2021; 74: 1109-1116.
18. Younossi ZM, Felix S, Jeffers T, et al. Performance of the enhanced liver fibrosis test to estimate advanced fibrosis among patients with nonalcoholic fatty liver disease. JAMA Netw Open 2021; 4(9): e2123923.
19. Stine JG, DiJoseph K, Pattison Z, et al. Exercise training is associated with treatment response in liver fat content by magnetic resonance imaging independent of clinically significant body weight loss in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Am J Gastroenterol 2023. doi.org/10.14309/ajg.0000000000002098.
20. Schneider CV, Zandvakili I, Thaiss CA, Schneider KM. Physical activity is associated with reduced risk of liver disease in the prospective UK Biobank cohort. JHEP Rep 2021; 3(3). 100263.
21. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019; 7: 776-785.
22. Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol 2023; 8: 179-191.
23. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31-39.
24. Anja G, Lefere S. Bariatric surgery for NAFLD: Indications and post-operative management. Clin Mol Hepatol 2022
25. Ng CH, Teng MLP, Chew NWS, et al. Statins decrease overall mortality and cancer related mortality but are underutilized in NAFLD: a longitudinal analysis of 12,538 individuals. Expert Rev Gastroenterol Hepatol 2022; 16: 895-901.
26. Cholankeril G, Kramer JR, Chu J, et al. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J Hepatol 2023; 78: 493-500.