Pituitary diseases: current screening and diagnostic approaches
Pituitary disease can lead to significant clinical consequences if not diagnosed and managed appropriately. Incidental pituitary lesions are increasingly encountered in clinical practice. The possibility of mass effect, as well as hormone hypersecretion and hyposecretion, need to be considered in patients with pituitary lesions. Primary practitioners have an important role in early diagnosis, initial workup and shared care alongside specialists. Expert pituitary multidisciplinary teams are essential for good patient outcomes.
- All patients with an incidental pituitary lesion, regardless of size or symptoms, should have a complete pituitary hormonal panel carried out.
- A pituitary-dedicated MRI is the preferred imaging modality for the diagnosis and monitoring of pituitary lesions.
- Formal visual field assessments are essential for patients with visual symptoms or with imaging findings suggestive of optic chiasm involvement.
- A diagnosis of hyperprolactinaemia should be established after two elevated prolactin levels are measured. An evaluation for macroprolactin, which may falsely elevate the prolactin level, should be performed. There are multiple differential causes of hyperprolactinaemia that need to be considered.
- Morning serum cortisol measurements are not useful for diagnosing hypercortisolism; screening should be performed using a 24-hour urine free cortisol test, a 1 mg dexamethasone suppression test or a late-night salivary cortisol assessment.
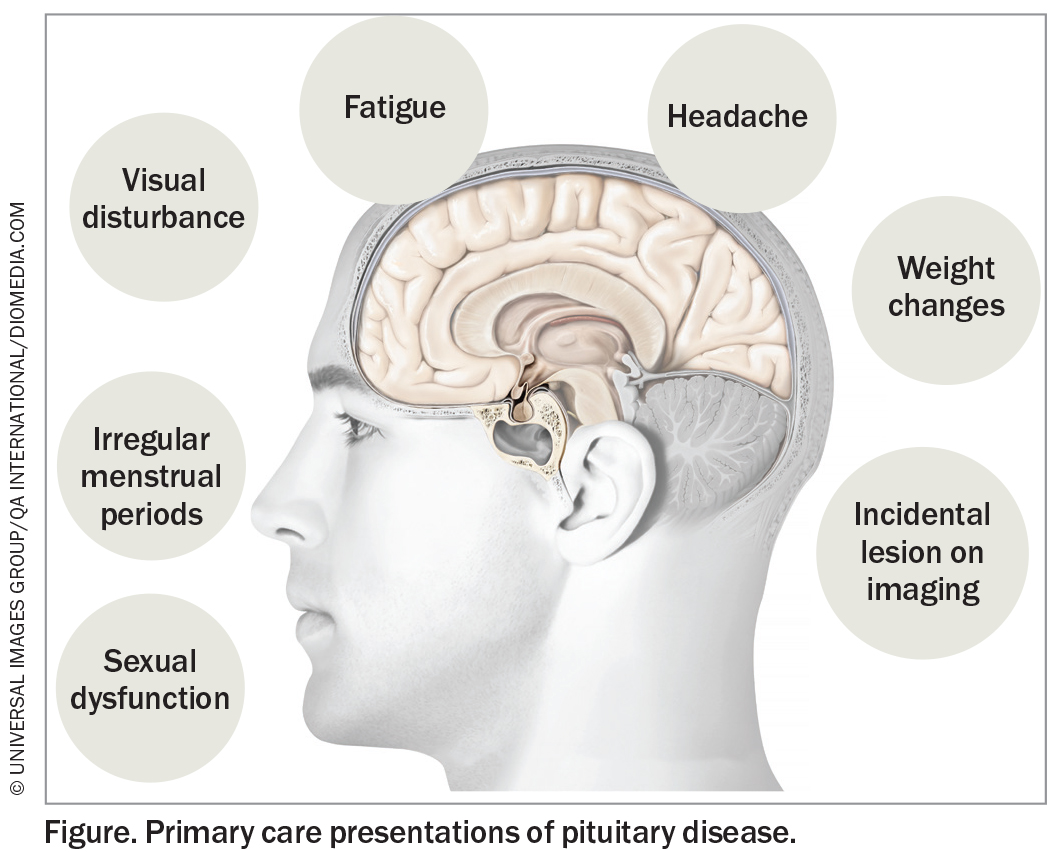
The pituitary gland plays a crucial role in the production of hormones targeting various endocrine organs and maintaining endocrine homeostasis. Overactivity and under-activity of the pituitary gland can cause a variety of pituitary disorders. Clinical presentation can be nonspecific and subtle, which may delay diagnosis. Therefore, a sound understanding of these types of presentations is essential to allow early identification and management of these conditions (Figure). This article reviews the current screening and diagnostic approaches for pituitary diseases.
How might patients present in a primary care setting?
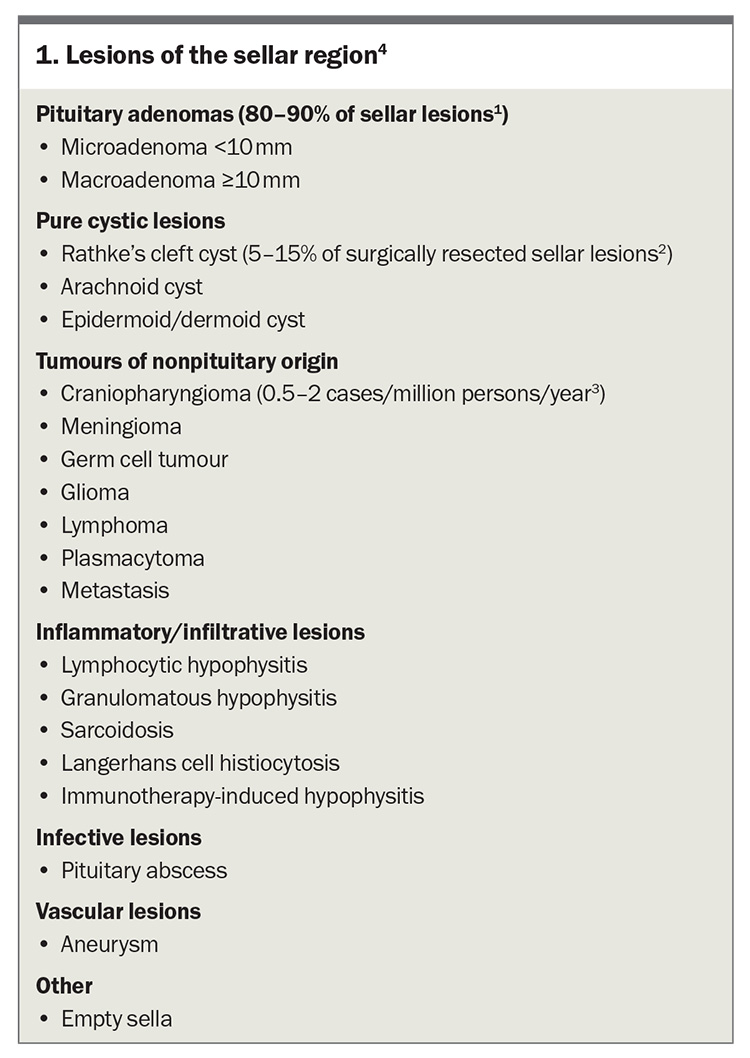
Patients with pituitary disease may present in primary care with an incidentally detected sellar lesion (Box 1), symptoms attributed to mass effect or clinical features of hormone hypersecretion or hormonal deficiency.1-4
Incidental detection of pituitary lesions
Incidental pituitary lesions are increasingly encountered in clinical practice because of increased utilisation of imaging and advances in neuroradiology. Pituitary incidentalomas are often identified during MRI studies performed for unrelated reasons, with a reported prevalence ranging from 10 to 20% in the healthy adult population.5 Microadenomas are more commonly encountered than macroadenomas.6,7 Most pituitary lesions are nonfunctioning pituitary adenomas or Rathke’s cleft cysts.
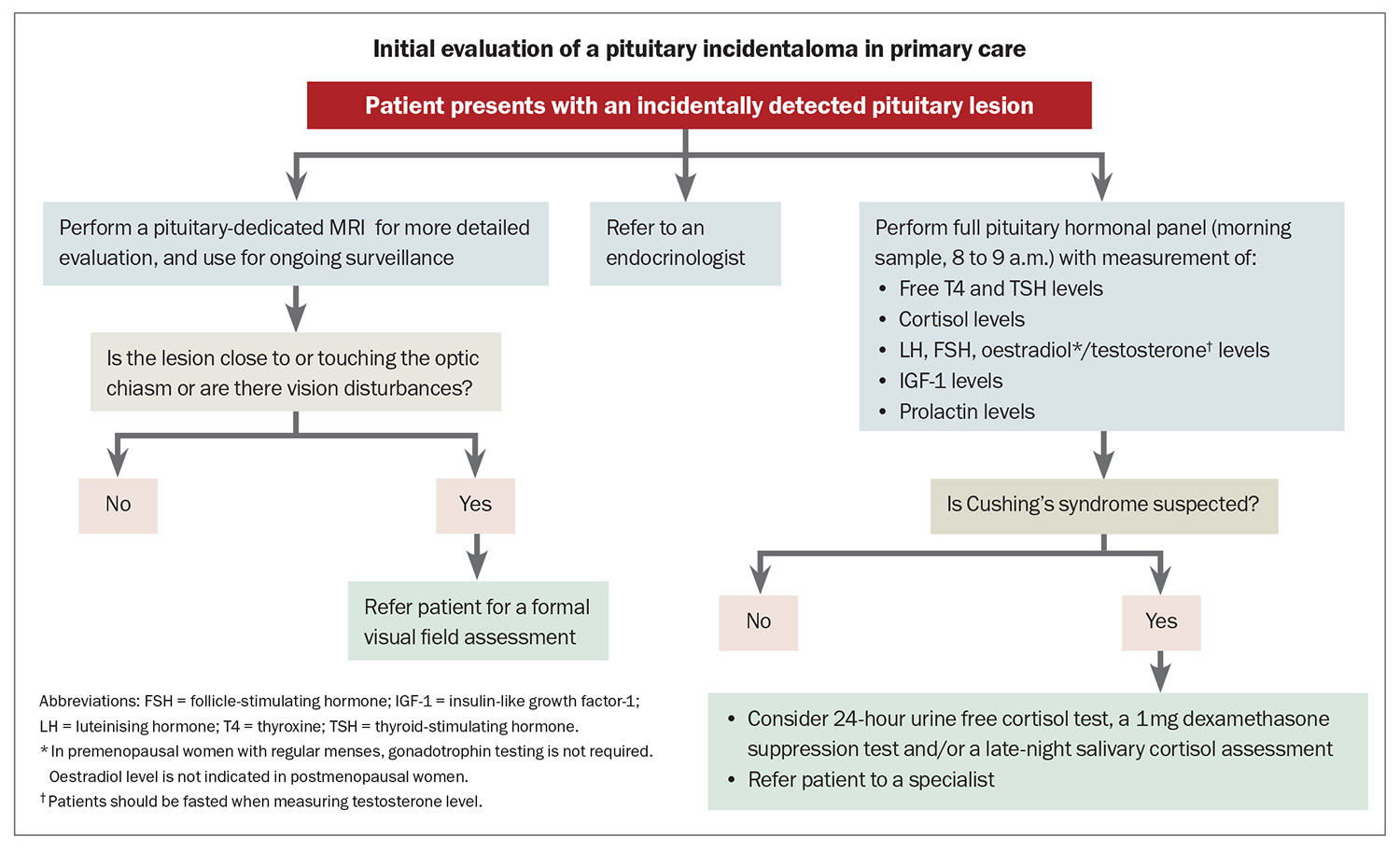
The Endocrine Society’s clinical practice guideline on pituitary incidentalomas recommends that all patients with a pituitary incidentaloma, even if asymptomatic, undergo clinical and biochemical evaluation for hormone dysfunction.8 A full pituitary hormonal panel will be adequate for most patients. If Cushing’s syndrome is suspected, further screening can be initiated in primary care, but this can also be performed by an endocrinologist (Flowchart). If the lesion was incidentally detected on CT imaging, a pituitary-dedicated MRI should be performed for more detailed evaluation, and used for ongoing surveillance.
Symptoms of mass effect
Visual field disturbances may arise when a pituitary tumour exerts contact or compression on the optic apparatus. Such disturbances are uncommon in microadenomas (<10 mm) and may serve as an indicator of a larger tumour. In these cases, patients may present with visual disturbances accompanied by other symptoms, such as headaches. Bedside visual assessment through confrontation testing can identify more pronounced deficits; however, formal visual field evaluation using Humphrey visual field testing is necessary for a comprehensive assessment.
Hormonal hypersecretion
Excessive production of pituitary hormones can result in a wide array of symptoms and signs, typically correlating with the specific actions of the overproduced hormone.
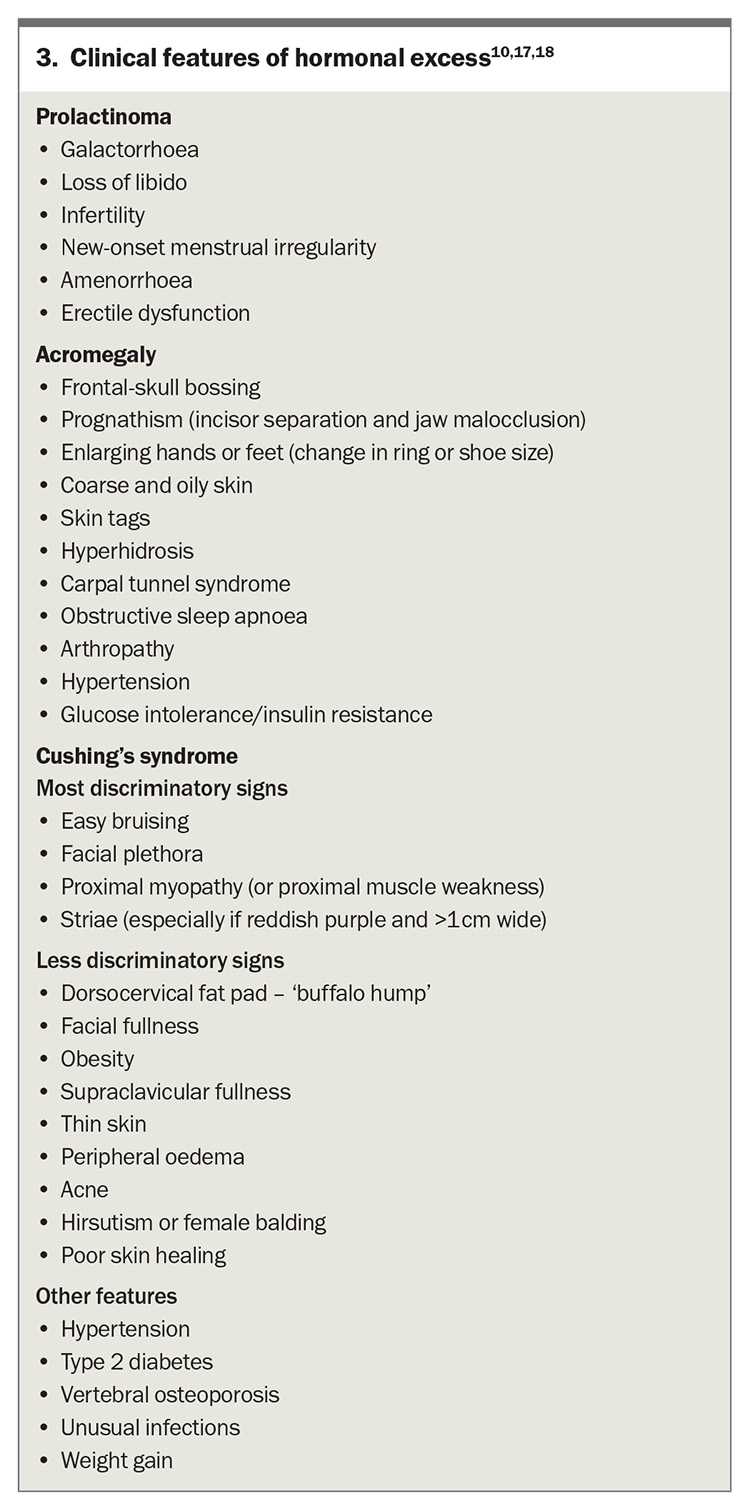
Prolactinoma
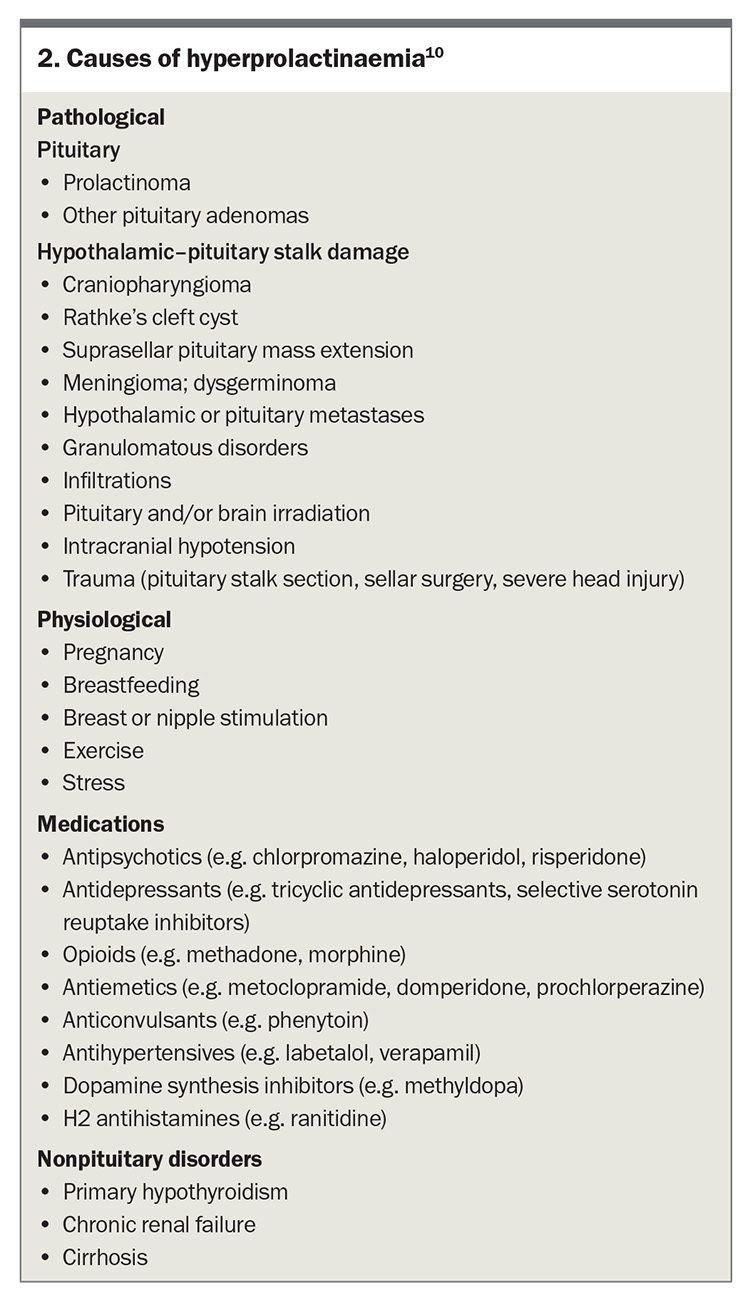
Prolactinoma is the most common functioning pituitary tumour accounting for about 50% of all pituitary tumours.9-11 It is essential to exclude other potential causes of elevated prolactin levels, which may include pregnancy, nipple stimulation and use of certain medications. Stress can also induce an elevation in prolactin levels (Box 2).
A diagnosis of hyperprolactinaemia should be established after two elevated prolactin levels are measured. If prolactin levels are more than 10 times the upper limit of normal, consider urgent repeat testing and early referral of the patient to an endocrinologist. If the stress of venepuncture is suspected to have affected the result, consider performing a cannulated prolactin test.10 Most laboratories will report on the presence of macroprolactin, which is a biologically inactive complex that can interfere with the prolactin assay. Hook-effect, occurring when high prolactin concentrations saturate assay antibodies, may falsely lower prolactin levels in the presence of a macroadenoma, but is uncommonly encountered with modern assays.12 In cases of large tumours that compress the pituitary stalk, elevated prolactin levels may result from interrupted negative feedback from the hypothalamus, a phenomenon known as the ‘stalk effect’. In such instances, the tumour may be either nonfunctioning or secreting a different hormone.
Presenting symptoms of a prolactinoma include oligomenorrhoea, amenorrhoea, galactorrhoea and infertility in women (Box 3).11 Tumours tend to be larger in men and are usually diagnosed in the setting of symptoms consistent with mass effect, such as visual disturbances and headaches, and less commonly because of decreased libido and erectile dysfunction. Historically, first-line management involved medical therapy with a dopamine agonist, such as cabergoline. However, the need for long-term therapy, increasing recognition of neuropsychiatric dopamine agonist adverse effects,13 and improvement in surgical technique have resulted in a recent international consensus statement suggesting consideration of surgical intervention as first-line therapy in select patients.10 Comprehensive counselling is mandatory within an experienced pituitary multidisciplinary team.
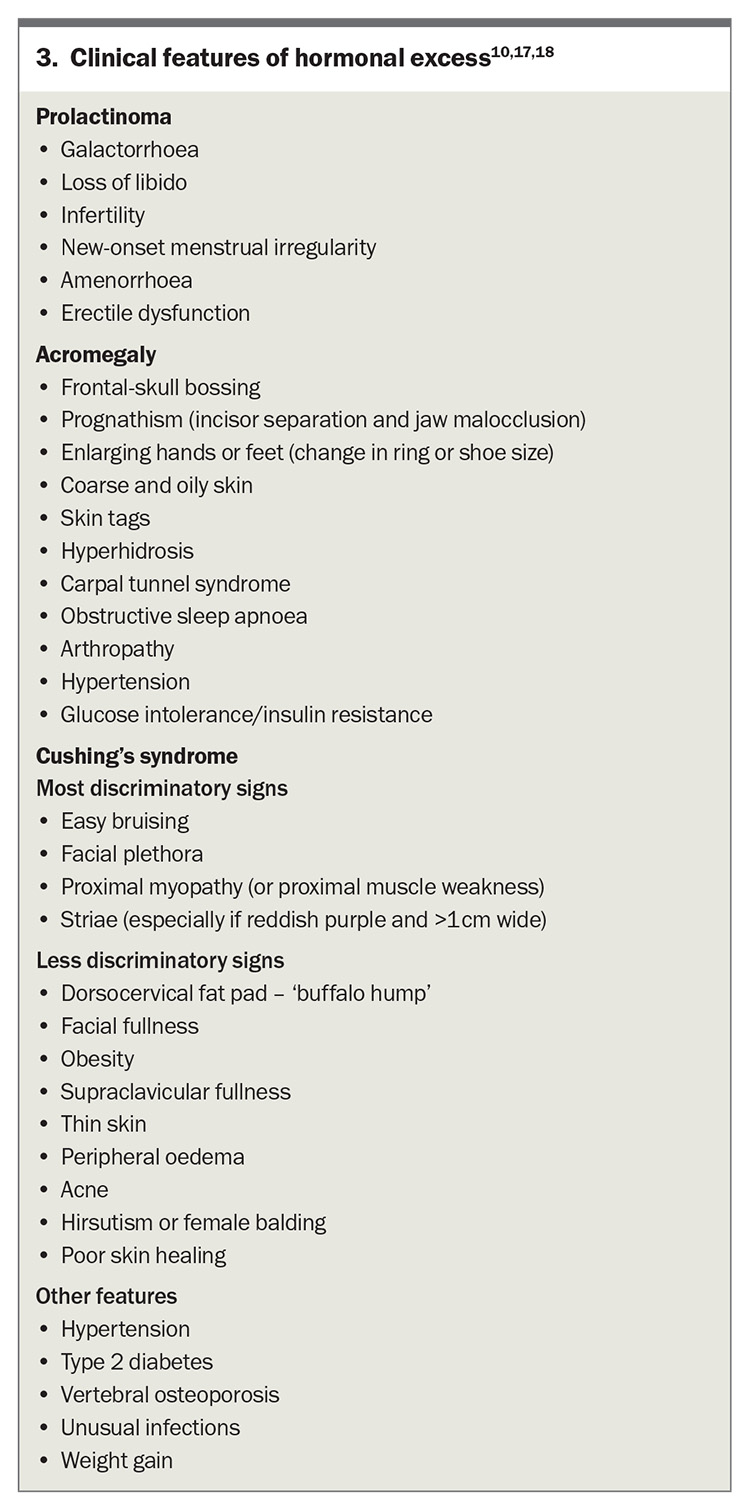
Acromegaly
Excess growth hormone production in adults leads to acromegaly, whereas growth hormone excess in childhood results in gigantism. This diagnosis should be considered in patients exhibiting typical clinical features of acromegaly, including enlarged hands and feet, jaw protrusion, frontal bossing, tongue enlargement and the presence of skin tags (Box 3). The combination of several associated comorbidities such as obstructive sleep apnoea, debilitating arthritis, type 2 diabetes mellitus, hypertension and carpal tunnel syndrome may also provide important diagnostic clues.14
The first-line investigation for acromegaly is the measurement of insulin-like growth factor-1 levels. Isolated or random serum growth hormone measurements are not reliable, as elevated levels may simply reflect the inherent pulsatile nature of growth hormone secretion. An oral glucose tolerance test may be useful to confirm growth hormone hypersecretion if it demonstrates lack of suppression of growth hormone; however, it is no longer recommended as part of the diagnostic workup.15 In healthy adults, growth hormone nadir level can be confounded by factors such as sex, body mass index (BMI) and the use of the oestrogen-containing oral contraceptive pill (OCP). It is appropriate to consider BMI-based growth hormone nadir cut-off values and to consider discontinuing use of the OCP for a minimum of four weeks before assessment.15
A growth-hormone secreting pituitary adenoma is present in more than 95% of patients with acromegaly.16 A pituitary MRI should be performed to detect a pituitary adenoma once biochemical confirmation has been achieved. Surgical resection of the tumour is the first-line treatment for acromegaly and may be curative if successful. Medical therapy is initiated in patients who are not candidates for surgical intervention. Periodic longitudinal evaluation and management of associated comorbidities are essential. The Endocrine Society’s clinical practice guideline on acromegaly also recommends screening colonoscopy at diagnosis and thyroid ultrasonography if there is palpable thyroid nodularity.14 Referral of the patient for endocrine review at the point of suspected acromegaly is appropriate. Surgery must be carried out within an expert pituitary centre.
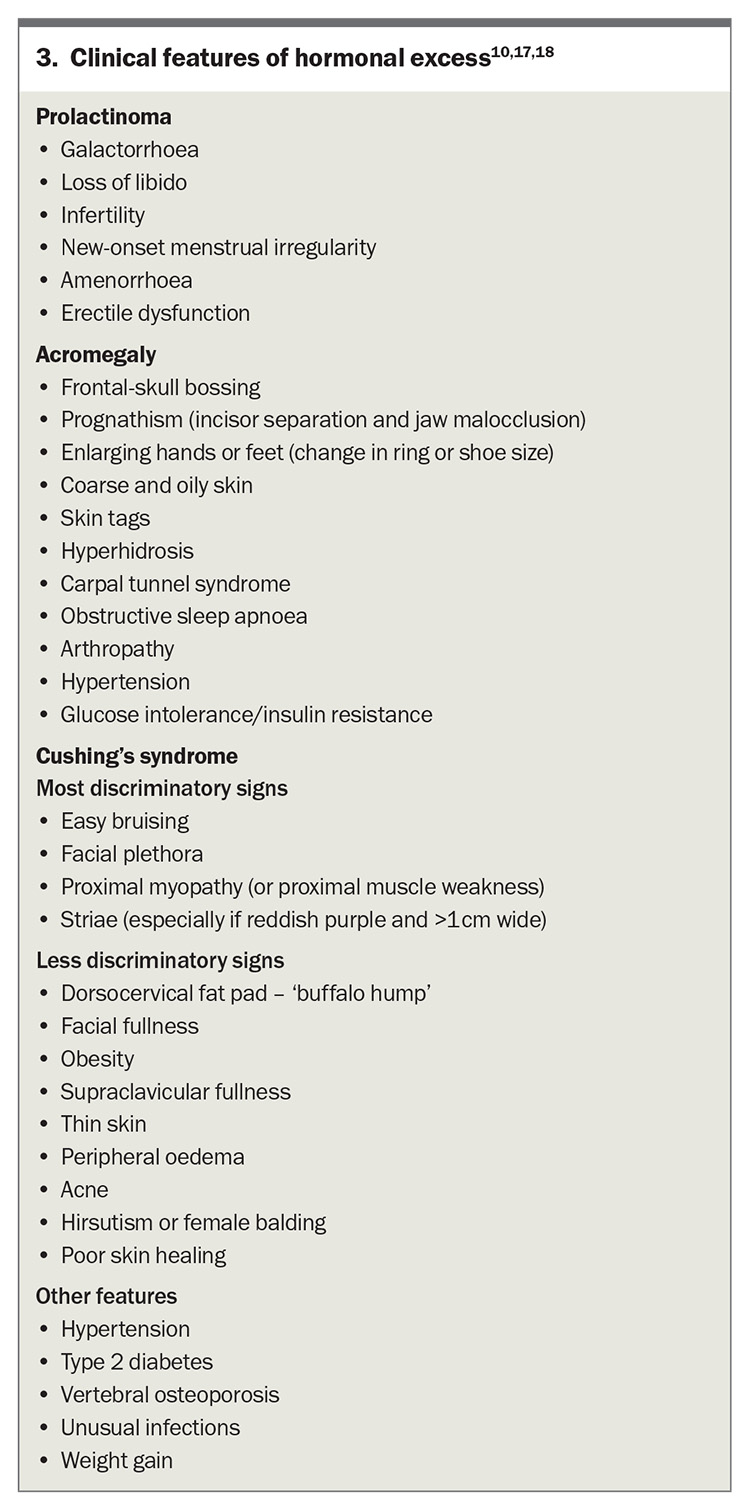
Cushing’s disease
Hypercortisolism of pituitary origin is referred to as Cushing’s disease whereas hypercortisolism of adrenal origin or ectopic source is termed Cushing’s syndrome. This can be a challenging diagnosis because many signs and symptoms can overlap with common conditions encountered in primary care and not all patients (e.g. obesity in isolation) will warrant routine screening. Patients displaying multiple and progressive features, particularly with clinical signs more suggestive of Cushing’s syndrome (Box 3) or features unusual for their age (e.g. osteoporosis), should be screened for Cushing’s syndrome.17,18
Morning measurement of cortisol levels cannot be used to diagnose Cushing’s syndrome. If there is sufficient clinical suspicion of Cushing’s syndrome, then appropriate screening tests include 1 mg overnight dexamethasone suppression test, 24-hour urine free cortisol test or late-night salivary cortisol test.
The complex workup required to diagnose Cushing’s disease is usually overseen by an endocrinologist with expertise in pituitary disorders, followed by first-line management in the form of surgical resection by a high-volume pituitary neurosurgeon.
It is crucial to exclude exogenous steroid use, including use of inhaled, topical or injected steroid medications, as a cause of cushingoid appearance before carrying out biochemical testing. Taking a thorough drug history is required so as not to overlook the use of skin creams and herbal medications containing glucocorticoid components.
Women taking the OCP have elevated cortisol-binding globulin levels, which increases total but not free cortisol levels. A 24-hour urine free cortisol test or late-night salivary cortisol test can be used for screening in this instance. If this level is normal and clinical suspicion is low, no further investigation is required. If ongoing concern, repeat the assessment at least six weeks after ceasing the OCP, if possible.
When performing a late-night salivary cortisol measurement, patients should be instructed to collect the saliva sample ideally before their usual bedtime, so as not to trigger a physiological cortisol response caused by the stress of forcing themselves to stay awake until midnight. Late-night salivary cortisol testing may not be appropriate for patients with an altered sleep-wake cycle, such as night-shift workers, as it assumes nadir of cortisol in late night.
If there is suspicion of Cushing’s syndrome, the patient should be referred to an endocrinologist, although screening can be initiated in primary care. A single abnormal screen may not be consistent with Cushing’s syndrome; conversely normal results in the right clinical circumstance may not exclude Cushing’s syndrome, for instance in the rare setting of cyclic Cushing’s syndrome. Additionally, delineating between a pituitary, adrenal or ectopic source of Cushing’s syndrome is challenging and best carried out in a specialist setting.
Other functional pituitary adenomas
Thyroid-stimulating hormone-secreting adenomas represent a rare entity in clinical practice, with a prevalence of less than 1% among all pituitary tumours.19 Functional gonadotroph adenomas are also infrequent, as the majority of gonadotroph tumours remain clinically silent.
Hormonal hyposecretion
Hypopituitarism
Hypopituitarism refers to inadequate secretion of one or more pituitary hormones. If impaired secretion of one hormone is identified, it is important to complete a full assessment of pituitary function because multiple hormonal axes may be affected. In patients with a pituitary tumour, the obstruction caused by the tumour or the increased pressure within the sellar can interfer with hypothalamic-releasing factors. Hypopituitarism could occur following surgical treatment of pituitary lesions and can occur many years after radiotherapy targeting the sella.20 Hypopituitarism can result from various other aetiologies including infiltrative or infectious disease, traumatic causes and use of certain medications (e.g. opioids, immune checkpoint inhibitors).21
Hypocortisolism
Secondary adrenal insufficiency is caused by adrenocorticotropic hormone (ACTH) deficiency. Symptoms of adrenal insufficiency can be nonspecific and may include fatigue, dizziness, nausea, abdominal pain and postural hypotension. A morning cortisol level measured at 8 to 9 a.m. is the first-line test for diagnosis,22 with a level of less than 100 nmol/L suggestive of hypocortisolism. Unlike primary adrenal insufficiency, patients with secondary adrenal insufficiency caused by pituitary disease have an inappropriately normal or low ACTH level. Although the short synacthen test is often used in assessing the pituitary–adrenal axis, its diagnostic utility in identifying secondary adrenal insufficiency may be limited in cases of recent onset. Hydrocortisone is the glucocorticoid replacement of choice, with total daily doses typically ranging from 15 to 20 mg, administered in divided doses, with a higher dose given in the morning to replicate physiological cortisol production. Patients should be educated about the necessity of stress dosing during periods of acute illness or surgery. It is also essential for patients to carry an emergency kit containing injectable glucocorticoids, along with an alert bracelet or card that outlines a documented sick-day management plan (available online at https://www.endocrinesociety.org.au/reseources-adrenal-insufficency-resources.asp).
Secondary hypothyroidism
Patients with secondary hypothyroidism may present with symptoms of hypothyroidism such as weight gain, fatigue and cold intolerance. Biochemical testing will show evidence of low free thyroxine and/or triiodothyronine levels with an inappropriately normal or low thyroid-stimulating hormone level. It is important to note that thyroid hormone accelerates the clearance of endogenous cortisol.23 Consequently, in a patient with newly diagnosed secondary hypothyroidism, it is crucial to exclude secondary adrenal insufficiency before initiating thyroid hormone replacement therapy. This is essential to prevent precipitating an adrenal crisis.
Secondary hypogonadism
Secondary hypogonadism is characterised by low-normal levels of follicle-stimulating hormone and luteinising hormone levels in the presence of reduced sex hormone levels. Factors such as obesity, excessive alcohol consumption and hyperprolactinaemia can contribute to the development of secondary hypogonadism. Congenital causes are rare and can include Kallman syndrome.24 In male patients, hypogonadism may present with features of testosterone deficiency, including reduced libido, erectile dysfunction and loss of body hair. Premenopausal women may have subfertility, amenorrhoea or oligomenorrhoea alongside biochemical evidence of low serum oestrogen levels. Hormonal replacement therapy in premenopausal women can significantly decrease standard mortality ratios25 and is recommended in accordance with established clinical guidelines.
Arginine-vasopressin deficiency
Arginine-vasopressin deficiency (AVP-D, previously referred to as central diabetes insipidus) typically presents with polyuria, nocturia, polydipsia and elevated serum osmolality. In patients with a pituitary adenoma, AVP-D is exceedingly rare, unless the patient has undergone pituitary surgery, thus presentation with AVP-D should lead to consideration of an alternative underlying pathology (e.g. a sellar metastasis). The serum sodium level can be in the high-normal range in untreated patients and it is unusual to develop hypernatraemia in patients with free access to water with an intact thirst sensation. Common causes of diuresis such as hyperglycaemia and hypercalcaemia should be excluded. Polyuria (urine output >50 mL/kg per day) should be confirmed with a 24-hour urine collection. Given that primary polydipsia and AVP resistance (previously termed nephrogenic diabetes insipidus) can present similarly, further evaluation often requires water deprivation, hypertonic saline or arginine stimulation testing. If clinically concerned, these patients should be referred for further endocrinological evaluation.
Growth hormone deficiency
Adult growth hormone deficiency is associated with significant morbidity and reduced quality of life.26 Measuring basal growth hormone levels is not useful in the diagnosis and in 20% of cases insulin-like growth factor-1 levels may be falsely normal.27 Confirmation of diagnosis requires confirmatory stimulation testing under endocrine guidance. Growth hormone replacement therapy is listed on the PBS for adults with proven growth hormone deficiency.
Pituitary apoplexy
Pituitary apoplexy occurs when there is acute haemorrhage into the pituitary gland or an infarction. Presentation is typically with sudden severe headache, sometimes with concurrent meningism, visual loss or cranial nerve palsy, and patients may not have a known pituitary adenoma previously. This constitutes an endocrine emergency as these patients may require immediate glucocorticoid replacement and consideration of surgical decompression. Referral of the patient to the emergency department should be considered to access urgent endocrine and neurosurgical care.28
Empty sella
Empty sella is characterised by flattening of pituitary tissue caused by herniation of the subarachnoid space within the sella turcica. In most cases it is a radiological finding without clinical implication. Growth hormone deficits and hyperprolactinaemia are the most commonly found associated pituitary hormonal abnormalities.29
Other lesions
Rathke’s cleft cysts are benign lesions that originate from the remnants of Rathke’s pouch. Although often asymptomatic, patients can experience headaches, visual disturbance and endocrine dysfunction.30 Craniopharyngiomas are rare brain tumours, arising from remnants of the Rathke’s cleft,31 that also present with symptoms of mass effect or hypopituitarism (including AVP-D) but often have characteristic imaging features. Management necessitates expert pituitary multidisciplinary teams for optimal patient outcomes.
Conclusion
Identification of pituitary disease can be challenging because there are a variety of clinical presentations that can be nonspecific and subtle. Understanding the different types of presentations is essential to allow early identification and management of these conditions and prevent significant clinical implications. Primary care physicians should have an appropriate index of suspicion and be familiar with the basic screening for hormonal abnormalities. Referral to an endocrinologist for diagnostic workup and direction of patient care to an expert pituitary multidisciplinary team is crucial for optimal patient outcome. ET
COMPETING INTERESTS: Dr Govinna: None. Professor McCormack has received research grants from Endocrine Society of Australia, Pfizer and IPSEN, consulting fees from Novo Nordisk and Recordati, payment from Novo Nordisk for production of an educational video, and support for attending meetings from Pfizer.
References
1.Molitch ME. Pituitary incidentalomas. Best Pract Res Clin Endocrinol Metab 2009; 23: 667-675.
2. Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N. Rathke’s cleft cysts. Clinical endocrinology 2012; 76: 151-160.
3. Müller HL. Craniopharyngioma. Endocr Rev 2014; 35: 513-543.
4. Kucharczyk W, Truwit CL. Diseases of the sella turcica and parasellar region. In: Hodler J, Kubik-Huch RA, von Schulthess GK, editors. Diseases of the brain, head and neck, spine 2020–2023: diagnostic imaging. Cham: Springer; 2020. p. 1.
5. Tahara S, Hattori Y, Suzuki K, Ishisaka E, Teramoto S, Morita A. An overview of pituitary incidentalomas: diagnosis, clinical features, and management. Cancers 2022; 14: 4324.
6. Westall SJ, Aung ET, Kejem H, Daousi C, Thondam SK. Management of pituitary incidentalomas. Clin Med 2023; 23: 129-134.
7. Yue N. Clinically serious abnormalities found incidentally at MR imaging of the brain: data from the Cardiovascular Health Study. Radiology 1997; 202: 41-46.
8. Freda PU. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011; 96: 894-904.
9. Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab 2009; 23: 543-554.
10. Petersenn S. Diagnosis and management of prolactin-secreting pituitary adenomas: a Pituitary Society international consensus statement. Nat Rev Endocrinol 2023; 19: 722-740.
11. Chanson P, Maiter D. The epidemiology, diagnosis and treatment of prolactinomas: the old and the new. Best Pract Res Clin Endocrinol Metab 2019; 33: 101290.
12. Raverot V, Perrin P, Chanson P, Jouanneau E, Brue T, Raverot G. The high-dose hook effect in prolactin immunoassay: does it still exist? Pituitary 2022; 25: 653-657.
13. De Sousa SMC, Baranoff J, Rushworth RL, et al. Impulse control disorders in dopamine agonist-treated hyperprolactinemia: prevalence and risk factors. J Clin Endocrinol Metab 2020; 105: dgz076
14. Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99: 3933-3951.
15. Giustina A, Biermasz N, Casanueva FF, et al. Acromegaly Consensus Group. Consensus on criteria for acromegaly diagnosis and remission. Pituitary 2024; 27: 7-22.
16. Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119: 3189-202.
17. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93: 1526-1540.
18. Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med 2020; 382: 937-950.
19. Tjörnstrand A, Nyström HF. Diagnostic approach to TSH-producing pituitary adenoma. Eur J Endocrinol 2017; 177: R183-R197
20. Rieken S, Habermehl D, Welzel T, et al. Long-term toxicity and prognostic factors of radiation therapy for secreting and non-secreting pituitary adenomas. Radiat Oncol 2013; 8: 18.
21. C JD. Anterior pituitary failure. In: The Pituitary. 3rd ed. Elsevier; 2011. p. 343-381.
22. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 3888-3921.
23. Beck-Peccoz P. Treatment of central hypothyroidism. Clin Endocrinol 2011; 74: 671-612.
24. Barber TM. Mechanisms of central hypogonadism. Int J Mol Sci 2021; 22: 8217.
25. Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. Lancet 2001; 357: 425-431.
26. Hoffman AR. Adult growth hormone deficiency: diagnostic and treatment journeys from the patients’ perspective. J Endocr Soc 2022; 6: bvac077
27. Biller BM, Samuels MH, Zagar A. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 2002; 87: 2067-2069.
28. Boyke A. Pituitary apoplexy: a re-appraisal of risk factors and best management strategies in the COVID-19 era. Pituitary 2024; doi: 10.1007/s11102-024-01420-0. Online ahead of print.
29. Chiloiro S, Giampietro A, Bianchi A, et al. Primary empty sella: a comprehensive review. Eur J Endocrinol 2017; 177: R275-R285..
30. Aydin S, Darko K, Detchou D, Barrie U. Rathke’s cleft cysts: from pathophysiology to management. Neurosurg Rev 2024; 47: 522.
31. Jane JA, Laws ER. Craniopharyngioma. Pituitary 2006; 9: 323-326.