Neuroendocrine neoplasms: time to widen the NET
Neuroendocrine neoplasms are a heterogeneous group of tumours that can present with nonfunctional symptoms or functional syndromes. Diagnosis is often delayed because of the nonspecific presentation. This article provides an update on the detection of neuroendocrine tumours and neuroendocrine carcinomas and the management of patients with these conditions.
- Most neuroendocrine neoplasms are indolent; however, a minority can behave aggressively.
- There is often significant delay between symptom onset and diagnosis because of the nonspecific nature of symptoms.
- Early recognition and diagnosis are crucial to allow patients to be treated before they develop more widespread disease.
- A wide range of therapeutic options are available for neuroendocrine neoplasms; more targeted therapies are being explored to optimise patient outcomes while limiting toxicity.
Neuroendocrine neoplasms (NENs) are a diverse range of tumours that originate from neuroendocrine cells in either endocrine or nonendocrine organs. NENs are heterogeneous in clinical features and histopathological appearance. They may produce vasoactive hormones or neurotransmitters.1,2 NENs of endocrine organs include medullary thyroid cancer, paragangliomas, phaeochromocytomas and pituitary neuroendocrine neoplasms. NENs may also occur in diffuse endocrine tissue in exocrine parenchyma, such as the gastroenteropancreatic system and lung. Historically, these NENs were classified as carcinoid tumours.3
NENs account for about 2% of malignant neoplasms.4 Their incidence is rising, which has been attributed to increased rates of incidental detection with improved radiological techniques and greater awareness.5 Despite the increased overall incidence of NENs, the rates of metastatic disease at presentation have remained largely stable.6 Diagnosis of NENs is often delayed because of the nonspecific symptoms. Multiple studies have reported a median time from initial symptoms to diagnosis of 36 to 58 months.7-9
This article focuses on NENs arising from common anatomical sites, including gastrointestinal, pancreatic, bronchopulmonary, thymic and genitourinary NENs. We discuss the presentation, diagnosis and management of patients with these NEN subtypes.
Classification of neuroendocrine neoplasms
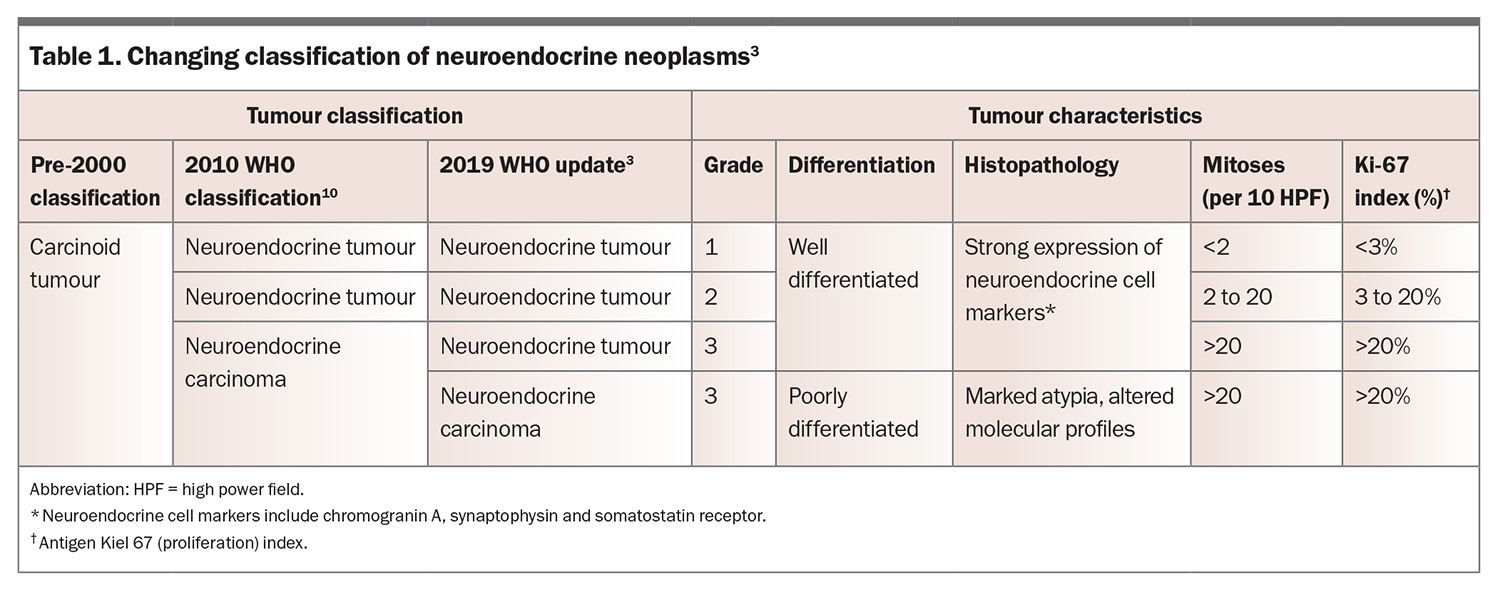
The classification of NENs has changed substantially over the past 25 years.3 Pre-2000, NENs of nonendocrine organs were classified as carcinoid tumours. In 2010, they were separated into neuroendocrine tumours (NETs) and neuroendocrine carcinomas, depending on their grade.10 In 2019, the WHO adjusted this classification system.3 Previously all grade 3 lesions were labelled neuroendocrine carcinoma, irrespective of the degree of differentiation. The 2019 update allowed for the existence of grade 3 well differentiated NETs, with only poorly differentiated tumours labelled neuroendocrine carcinoma (Table 1).3,11
Well-differentiated lesions with molecular and morphological features consistent with cells of neuroendocrine origin are classified as NETs. These strongly express markers of neuroendocrine cells, including chromogranin A, synaptophysin and somatostatin receptors.11 In contrast, neuroendocrine carcinomas comprise cells with marked atypia and altered molecular profiles. They retain sufficient evidence of neuroendocrine cell differentiation, but this is significantly less prominent compared with NETs.3,12 Neoplasms with mixed neuroendocrine and epithelial components have also been identified, termed mixed neuroendocrine and non-neuroendocrine neoplasms.13
Genetics
Most NENs are sporadic. However, some NENs (fewer than 5%) are associated with a genetic condition, such as multiple endocrine neoplasia type 1 or 2, von Hippel-Lindau disease, tuberous sclerosis, neurofibromatosis or Mahvash disease.14-16 Genomic analyses have demonstrated a complex mutational milieu with certain NENs.
Signs and symptoms
NENs can be functional or nonfunctional. Improved radiological techniques have led to increasing numbers of incidentally found NENs, which can be asymptomatic. If an NEN is functional, the clinical syndrome depends on the hormone secreted. Paraneoplastic syndromes such as tumour-induced hypercalcaemia, syndrome of inappropriate antidiuretic hormone, ectopic Cushing’s syndrome and autoimmune neuropathies or encephalomyelitis can occur.17,18
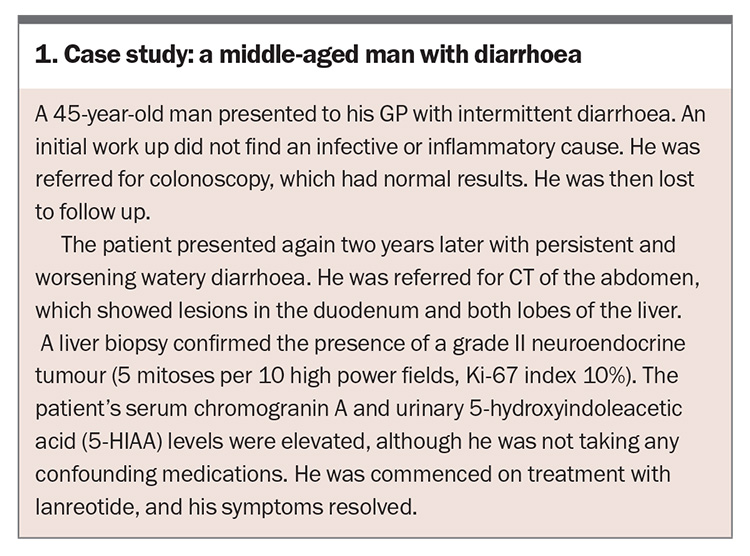
Patient presentation varies depending on the organ of origin of the NEN. Signs and symptoms are often nonspecific, leading to delays in diagnosis, as illustrated in the case study (Box 1).9 Many patients are misdiagnosed with conditions such as irritable bowel syndrome, dyspepsia, inflammatory bowel disease, menopause, asthma or anxiety following reviews in primary and secondary care.7,19
Subtypes of neuroendocrine neoplasm
Gastrointestinal NENs
Gastrointestinal NENs can affect the stomach, small bowel, appendix and colon. They can cause symptoms of mass effect, including abdominal discomfort or constipation.
The small intestine is a common site for NENs. Carcinoid syndrome occurs in a minority of patients with metastatic disease.20 Although the primary tumour may be small, associated mesenteric lymphadenopathy can cause fibrosis leading to bowel obstruction and ischaemia.21 A prospective study suggested that age greater than 60 years, male sex, body mass index greater than 35 kg/m2 and use of menopausal hormone therapy were potentially associated with the development of small intestine NEN.22 A family history of cancer was not statistically significant in this study. In contrast, a population- based study found patients with a first-degree relative with small intestine NEN had a higher incidence of developing the disease themselves.23
Carcinoid syndrome occurs most commonly with midgut and bronchopulmonary or thymic NENs. In a database review of 9512 patients with NENs, 19% had carcinoid syndrome.24 Characteristic symptoms of carcinoid syndrome include facial flushing, diarrhoea and shortness of breath.25 Acute complications include carcinoid crisis, which presents with hypotension, tachycardia, flushing and bronchospasms and typically occurs after anaesthesia or surgery, but can occur with no trigger. Longer term complications are related to fibrosis, causing mesenteric or retroperitoneal fibrosis or carcinoid heart disease.26 The occurrence of carcinoid syndrome has been significantly associated with tumour grade, stage and primary tumour site, and is associated with a lower overall survival rate.24 It predominantly occurs in the setting of liver or bone metastases, where serotonin metabolites and other hormones and proteases are able to bypass the portal vein circulation.25
Gastric NENs are classified into four groups:14,27
- type I gastric NENs, arising from enterochromaffin cells affected by chronic atrophic gastritis, which can be associated with pernicious anaemia
- type II gastric NENs, which have been associated with multiple endocrine neoplasia 1 or Zollinger-Ellison syndrome and are characterised by oversecretion of gastrin
- type III gastric NENs, which do not produce gastrin and are typically sporadic, large, invasive lesions that behave aggressively despite being well differentiated; they can cause a histamine-mediated atypical carcinoid syndrome leading to a pruritic, erythematous and patchy rash
- type IV gastric NENs, which are poorly differentiated carcinomas.
Colorectal NENs are is rarer than small intestinal NENs are. It has a tendency to behave more aggressively and can have a poorer prognosis than adenocarcinoma.28
Pancreatic NENs
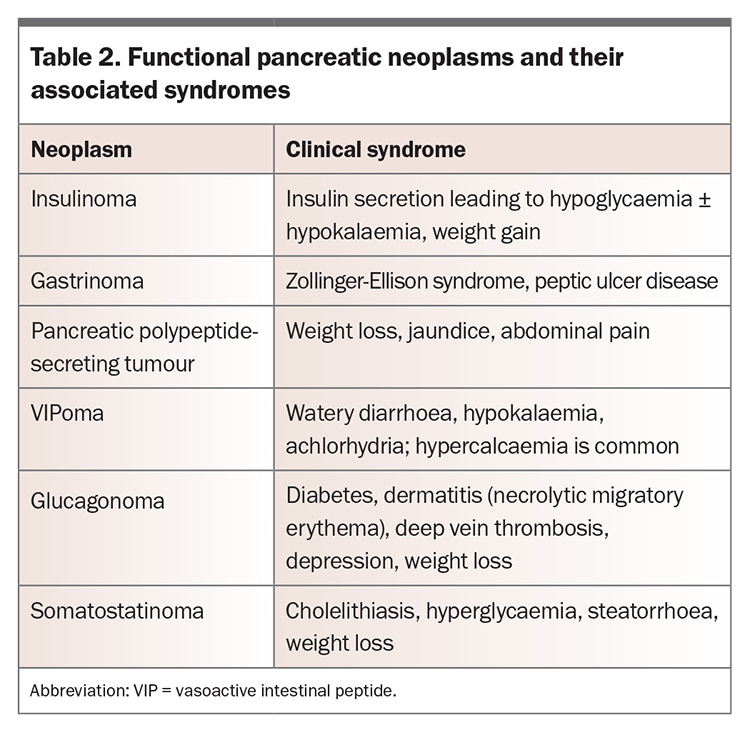
Pancreatic NENs arise from pancreatic islet cells. Most pancreatic NENs are nonfunctional, but functional tumours can occur. The clinical syndrome relates to the specific hormone secreted (Table 2). Pancreatic NENs can secrete multiple hormones or initially present as nonfunctional and subsequently progress to hormone production. Locally advanced disease can cause nonhormonal symptoms such as pain, biliary obstruction, pancreatitis or intestinal obstruction.29 Distant metastatic disease can cause symptoms that depend on the organ involved. A systematic review found that smoking and a first-degree family history of cancer were significantly associated with the development of sporadic pancreatic NEN; however, the data were insufficient to support smoking or alcohol intake as risk factors.30
Bronchopulmonary and thymic NENs
Bronchopulmonary and thymic NENs are divided into four groups: typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma and small-cell lung carcinoma. These groups differ in their histopathology, clinical behaviour and prognosis.14,31 Most lung NENs are small cell lung cancer.
Smoking is significantly associated with the development of small cell lung cancers and large cell neuroendocrine carcinomas, which are more common in older men. Small cell lung cancer is particularly aggressive and has a poor prognosis. Carcinoid tumours occur typically in women aged 40 to 50 years, and smoking is thought to be less of a risk factor.31 Although small cell lung cancer typically presents as a central lesion, large cell neuroendocrine carcinoma tends to be more peripheral. Central lesions can present with chest pain, haemoptysis, dyspnoea, pain and recurrent pneumonia, whereas peripheral lesions are often found incidentally.
Thymic NENs are rare. A significant proportion are associated with multiple endocrine neoplasia type 1.32
Genitourinary NENs
NENs affecting the kidney or bladder are rare. They can present with abdominal or flank pain, haematuria or weight loss.33 Most genitourinary NENs are high grade.34
Investigations
Serum chromogranin A is a measurable screening biomarker in the setting of NEN. However, the reported sensitivity and specificity of this biomarker vary significantly between different NENs (sensitivity, 46 to 100%; specificity 68 to 100%).35,36 Poorer survival outcomes have been correlated with increased levels of circulating chromogranin A.37 Chromogranin A levels can also be affected by other conditions, including cardiac disease, inflammatory disorders, atrophic gastritis and use of medications such as proton pump inhibitors and histamine antagonists. This affects the utility of chromogranin A in diagnosis and serial monitoring of patients with NEN in the real world setting.38
Urinary 5-hydroxyindoleacetic acid (5-HIAA), the metabolite of serotonin, can be measured over 24 hours patients with suspected carcinoid syndrome to diagnose and subsequently monitor response to treatment.38 Reported sensitivity and specificity vary depending on the type of NEN, and notably the test can have negative results even in the presence of clinically evident carcinoid syndrome.
Diagnosis
Tissue sampling of NENs via biopsy or resection is crucial for both diagnosis and management. Levels of hormones relating to specific functional syndromes can also be measured.
Various imaging modalities are used to help localise and stage NENs. Conventional techniques such as CT and MRI can be used to localise tumours.39 Endoscopic ultrasound examination has demonstrated superiority to CT and MRI imaging for the detection of pancreatic NENs and also enables tissue biopsy.40
Functional imaging, including fluorine-18 (e.g. FDG) and gallium-68 DOTATATE positron emission tomography (PET), plays a complementary role, particularly when deciding on appropriate therapeutic options such as peptide receptor radionuclide therapy.41 NENs express somatostatin receptors, and DOTATATE PET can facilitate their detection, especially for NENs that are well differentiated. As NENs become more poorly differentiated, their expression of somatostatin receptors reduces and they show reduced avidity on DOTATATE imaging. This change is associated with increasing proliferation rates and metabolic activity, which can be detected on FDG-PET imaging.42
Role of the GP in diagnosis of NENs
Given the high rates of delayed diagnosis and misdiagnosis, GPs can play a central role in the initial detection of NENs. The symptoms of NENs are nonspecific; however, clinical suspicion should be triggered if these common symptoms are unexplained, persistent or severe. First-line investigations that can be ordered include baseline biochemistry and structural imaging. Measurement of serum chromogranin A and urinary 5-HIAA levels may be helpful, noting the significant chance of false-positive results caused by use of medications or other medical conditions. Depending on the results, the patient may require bronchoscopy or gastroscopy and colonoscopy. Timely referral to the relevant specialist, such as a medical oncologist, respiratory physician, gastroenterologist or endocrinologist, should be considered. Optimal care pathways for patients with NENs in Australia were published in 2022 (www.cancer.org.au/health-professionals/optimal-cancer-care-pathways; www.cancer.org.au/assets/pdf/neuroendocrine-tumours-1st-edition).43
Management
Given the complexity and heterogeneity of NENs, a multidisciplinary team approach is required for management, including medical oncology, radiation oncology, surgery, endocrinology, anatomical pathology, nuclear medicine and radiology.44 Specialised nursing staff play a key role in management. Psychosocial support through allied health services and psychologists can be key for patients with NENs who experience anxiety and depression around their diagnosis.45 Patient support and advocacy organisations such as NeuroEndocrine Cancer Australia (NECA) may be helpful to patients and their carers (https://neuroendocrine.org.au).
Local disease
The mainstay of treatment for local disease is surgical or endoscopic resection when possible, particularly in the setting of stage I to III disease. Recurrence rates after resection range from 10 to 26%. A study found the median disease-free survival for pancreatic NEN was 52.1 months for metastatic disease and 97.4 months for regional disease.46 Given the possibility of late recurrence, longer monitoring periods may need to be considered.
Metastatic disease
Hepatic metastases of NENs are common. Liver-directed therapies include ablation, transarterial embolisation and transarterial cheomoembolisation.47 Selective internal radiation therapy with yttrium-90 or surgical resection can also be considered.48
Somatostatin analogues
Somatostatin analogues such as octreotide and lanreotide were shown to reduce or stabilise the growth of NENs in the PROMID and CLARINET trials.49,50 Somatostatin analogues are the most commonly used first-line treatment for patients with grade I or II NET; however, alternatives should be considered for patients with more aggressive or higher-grade disease.51 Active surveillance can be considered for patients with low-volume and nonfunctional NENs.
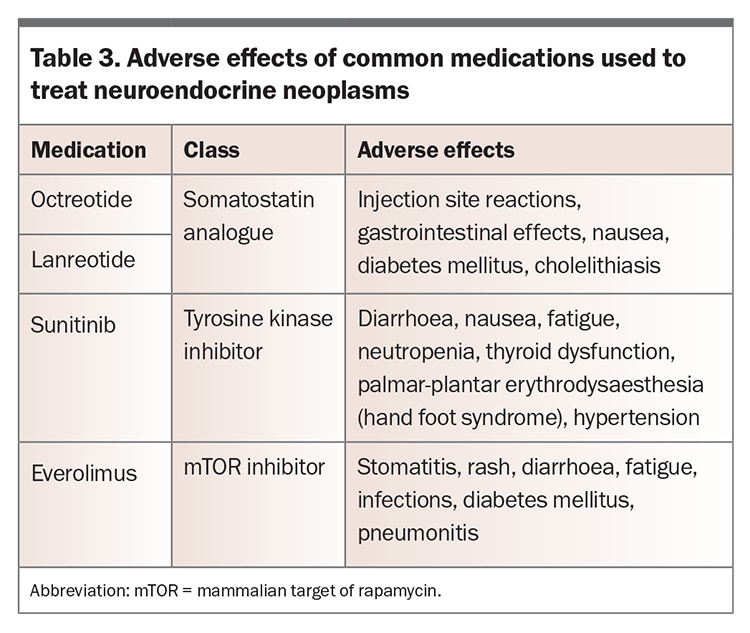
In the management of carcinoid syndrome, avoiding triggers and ensuring alternative contributors to diarrhoea have been managed are key. Somatostatin analogues such as octreotide and lanreotide can be used to reduce the severity of symptoms as well as improve urinary 5-HIAA levels. Adverse effects of octreotide and lanreotide are summarised in Table 3.52
Peptide receptor radionuclide therapy
The peptide receptor radionuclide lutetium (177Lu-DOTATATE) is used in treatment of patients with NETs. It targets the tumour with the combination of a radionuclide and a peptide somatostatin analogue that attaches to a surface receptor on the NET.53 Given the selectivity of this treatment for NETs, it is generally well tolerated.
The NETTER-1 phase III trial concluded that peptide receptor radionuclide therapy effectively slows disease progression, provides symptom relief and reduces tumour size.54 This study included participants whose condition had progressed on octreotide treatment. Most had undergone primary resection or had a grade 1 tumour. The NETTER-2 trial showed that a peptide receptor radionuclide used as first-line treatment was associated with improved progression-free survival and objective response rate in patients with high-grade advanced gastroenteropancreatic NETs.55
Major adverse effects of peptide receptor radionuclide therapy include nausea, acute kidney injury, transient bone marrow suppression, myelodysplastic syndrome and acute myeloid leukaemia.56 Patient suitability for peptide receptor radionuclide therapy is determined by the degree of DOTATATE avidity of known tumour lesions.
Molecular therapies
The molecular therapies sunitinib and everolimus have been used in patients with advanced NENs. Sunitinib is a small molecule tyrosine kinase inhibitor that blocks pathways that promote carcinogenesis in NENs, and everolimus is an inhibitor of mammalian target of rapamycin (mTOR). Improvements in progression-free survival but not quality of life were shown with sunitinib.57 The RADIANT-3 and RADIANT-4 trials compared everolimus with placebo and showed improved progression-free survival in patients with pancreatic NENS and gastrointestinal and lung NENs, respectively. Adverse effects of sunitinib and everolimus are summarised in Table 3.58,59
Chemotherapy
Various chemotherapy regimens, such as capecitabine and temozolomide (CAPTEM), carboplatin and etoposide or cisplatin and etoposide, can be used for patients with advanced NET and neuroendocrine carcinoma. Chemotherapy typically has greater efficacy against rapidly dividing tumours as opposed to the indolent pattern of growth typical of NETs.60 However, CAPTEM has been reported to be effective in patients with lower grade pancreatic NENs as well as grade 3 NENs.61
Surveillance
Active surveillance with monitoring of biochemistry and imaging results can be considered in certain clinical scenarios, such as patients with small, nonfunctional and low-grade pancreatic or small bowel NENs.62,63 However, there are no consensus guidelines regarding the optimal imaging modality and frequency of monitoring, nor the role of chromogranin A measurement in this setting. The decision to pursue surveillance versus active treatment should also consider the patient’s treatment preference, tumour burden and comorbidities.
Role of the GP in management of NENs
A shared care model for NENs includes specialist and primary care physicians in addition to specialty nurses and allied health professionals. The benefit of GP care for cancer survivors is well documented.64 GPs can monitor for adverse effects of treatment between specialist appointments. GPs also play a central role in communication with the patient and their family, as well as providing individualised psychosocial support and referral to psychological and allied health services or support and advocacy groups. Resources on NENs for GPs and patients are listed in Box 2.
Prognosis
A 2017 study in the US found the median overall survival across all NEN types and stages to be 112 months.65 However, the stage and grade of disease are the main determinants of survival, as observed in local and international studies.4,5 Low-grade disease may never recur after resection, whereas patients presenting with high-grade metastatic neuroendocrine carcinoma may have a significantly guarded prognosis.
Conclusion
NENs are a heterogeneous group of tumours affecting a wide range of body systems, which are increasing in incidence. Given the frequent delay between symptom onset and diagnosis, improved awareness of these conditions is key, to facilitate earlier diagnosis and optimise patient outcomes. Therapeutic options continue to evolve and include more targeted therapies that reduce adverse effects and maximise outcomes for patients. ET
COMPETING INTERESTS: Dr Chan has received advisory fees or honoraria from Ipsen and Camurus and was involved in the development of resources for NeuroEndocrine Australia. Dr Majumber, Associate Professor Tsang: None.
References
1. Hofland J, Kaltsas G, de Herder WW. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr Rev 2020; 41: 371-403.
2. Perren A, Couvelard A, Scoazec JY, et al; Antibes Consensus Conference participants. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pathology: diagnosis and prognostic stratification. Neuroendocrinology 2017;105: 196-200.
3. Rindi G, Mete O, Uccella S, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol 2022; 33: 115-154.
4. Reeders J, Ashoka Menon V, Mani A, et al. Clinical profiles and survival outcomes of patients with well-differentiated neuroendocrine tumors at a health network in New South Wales, Australia: retrospective study. JMIR Cancer 2019; 5: e12849.
5. Wyld D, Wan MH, Moore J, et al. Epidemiological trends of neuroendocrine tumours over three decades in Queensland, Australia. Cancer Epidemiol 2019; 63: 101598.
6. Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015; 121: 589-597.
7. Basuroy R, Bouvier C, Ramage JK, et al. Presenting symptoms and delay in diagnosis of gastrointestinal and pancreatic neuroendocrine tumours. Neuroendocrinology 2018; 107: 42-49.
8. Basuroy R, Bouvier C, Ramage JK, et al. Delays and routes to diagnosis of neuroendocrine tumours. BMC Cancer 2018; 18: 1122.
9. Singh S, Granberg D, Wolin E, et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J Glob Oncol 2016; 3:43-53.
10. Bosman F, Carneiro F. World Health Organization classification of tumours, pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2010.
11. Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med 2023; 12: 5138.
12. Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010; 39: 707-712.
13. Brathwaite SA, Smith SM, Wai L, et al. Mixed adenoneuroendocrine carcinoma: a review of pathologic characteristics. Hum Pathol 2018; 73: 184-191.
14. Oronsky B, Ma PC, Morgensztern D, et al. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia 2017; 19: 991-1002.
15. Hendifar AE, Dhall D, Strosberg JR. The evolving treatment algorithm for advanced neuroendocrine neoplasms: diversity and commonalities across tumor types. Oncologist 2019; 24: 54-61.
16. Giusti F, Marini F, Tonelli F, et al. Multiple endocrine neoplasia type 1. In: Bilezikian JP, Martin TJ, Clemens TL, Rosen CJ, editors. Principles of bone biology. 4th ed. Academic Press; 2020: 1293-1306.
17. Soomro Z, Youssef M, Yust-Katz S, et al. Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis 2020; 12: 6253-6263.
18. Guilmette J,Nosé V. Paraneoplastic syndromes and other systemic disorders associated with neuroendocrine neoplasms. Semin Diagn Pathol 2019; 36: 229-239.
19. Raphael MJ, Chan DL, Law C, et al. Principles of diagnosis and management of neuroendocrine tumours. CMAJ 2017; 189: e398-e404.
20. Howe JR, Cardona K, Fraker DL, et al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017; 46: 715-731.
21. Scott AT, Howe JR. Management of small bowel neuroendocrine tumors. J Oncol Pract 2018; 14: 471-482.
22. Cross AJ, Hollenbeck AR, Park Y. A large prospective study of risk factors for adenocarcinomas and malignant carcinoid tumors of the small intestine. Cancer Causes Control 2013; 24: 1737-1746.
23. Kharazmi E, Pukkala E, Sundquist K, et al. Familial risk of small intestinal carcinoid and adenocarcinoma. Clin Gastroenterol Hepatol 2013; 11: 944-949.
24. Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol 2017; 18: 525-534.
25. Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo) 2018; 73(Suppl 1): e490s.
26. Gade AK, Olariu E, Douthit NT. Carcinoid syndrome: a review. Cureus 2020; 12: e7186.
27. Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol 2020; 12: 791-807.
28. Niederle MB, Hackl M, Kaserer K, et al. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer 2010; 17: 909-918.
29. Ito T, Masui T, Komoto I, et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol 2021; 56: 1033-1044.
30. Haugvik SP, Hedenström P, Korsæth E, et al. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology 2015; 101: 133-142.
31. Leoncini E, Carioli G, La Vecchia C, et al. Risk factors for neuroendocrine neoplasms: a systematic review and meta-analysis. Ann Oncol 2016; 27: 68-81.
32. Yliaska I, Tokola H, Ebeling T, et al. Thymic neuroendocrine tumors in patients with multiple endocrine neoplasia type 1. Endocrine 2022; 77: 527-537.
33. Virarkar M, Vulasala SS, Gopireddy D, et al. Neuroendocrine neoplasms of the female genitourinary tract: a comprehensive overview. Cancers (Basel) 2022; 14: 3218.
34. Le BK, McGarrah P, Paciorek A, et al. Urinary neuroendocrine neoplasms treated in the "modern era": a multicenter retrospective review. Clin Genitourin Cancer 2023; 21: 403-414.
35. Gut P, Czarnywojtek A, Fischbach J, et al. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci 2016; 12: 1-9.
36. Rossi RE, Ciafardini C, Sciola V, et al. Chromogranin A in the follow-up of gastroenteropancreatic neuroendocrine neoplasms: is it really game over? A systematic review and meta-analysis. Pancreas 2018; 47: 1249-1255.
37. Marotta V, Zatelli MC, Sciammarella C, et al. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer 2018; 25: R11-R29.
38. Oberg K, Couvelard A, Delle Fave G, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: biochemical markers. Neuroendocrinology 2017; 105: 201-211.
39. Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: an update for the clinician. Int J Endocr Oncol 2015; 2: 159-168.
40. Ishii T, Katanuma A, Toyonaga H, et al. Role of endoscopic ultrasound in the diagnosis of pancreatic neuroendocrine neoplasms. Diagnostics (Basel) 2021; 11.
41. Chan DL, Hayes AR, Karfis I, et al. Dual [68Ga]DOTATATE and [18F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: a multicentre validation of the NETPET score. Br J Cancer 2023; 128: 549-555.
42. Hayes AR, Furtado O’Mahony L, Quigley AM, et al. The combined interpretation of 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT in metastatic gastroenteropancreatic neuroendocrine tumors: a classification system with prognostic impact. Clin Nucl Med 2022; 47: 26-35.
43. Cancer Council Victoria. Optimal care pathway for people with neuroendocrine tumours. 1st edition. Melbourne: Cancer Council Victoria, Australian Government; 2022. Available online at: www.cancer.org.au/assets/pdf/neuroendocrine-tumours-1st-edition (accessed April 2024).
44. Magi L, Mazzuca F, Rinzivillo M, et al. Multidisciplinary management of neuroendocrine neoplasia: a real-world experience from a referral center. J Clin Med 2019; 8: 910.
45. Song L, Cao Y, Li J, et al. Psychological distress and resilience in patients with gastroenteropancreatic neuroendocrine tumor. Front Endocrinol (Lausanne) 2022; 13: 947998.
46. Chan H, Zhang L, Choti MA, et al. Recurrence patterns after surgical resection of gastroenteropancreatic neuroendocrine tumors: analysis from the National Comprehensive Cancer Network Oncology Outcomes Database. Pancreas 2021; 50: 506-512.
47. Cazzato RL, Hubelé F, De Marini P, et al. Liver-directed therapy for neuroendocrine metastases: from interventional radiology to nuclear medicine procedures. Cancers (Basel) 2021; 13: 6368.
48. Lehrman ED, Fidelman N. Liver-directed therapy for neuroendocrine tumor liver metastases in the era of peptide receptor radionuclide therapy. Semin Intervent Radiol 2020; 37: 499-507.
49. Rinke A, Wittenberg M, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology 2017; 104: 26-32.
50. Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014; 371: 224-233.
51. Eads JR, Halfdanarson TR, Asmis T, et al. Expert consensus practice recommendations of the North American Neuroendocrine Tumor Society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. EndocrRelat Cancer 2023; 30: e22020.
52. Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res 2010; 29: 19.
53. Del Olmo-García MI, Prado-Wohlwend S, Bello P, et al. Peptide receptor radionuclide therapy with [(177)Lu]Lu-DOTA-TATE in patients with advanced GEP NENS: present and future directions. Cancers (Basel) 2022; 14: 584.
54. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N EnglJMed2017; 376: 125-135.
55. Singh S, Halperin DM, Myrehaug S, et al. [177Lu]Lu-DOTA-TATE in newly diagnosed patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: primary analysis of the phase 3 randomized NETTER-2 study. J Clin Oncol 2024; 42: LBA588-LBA588.
56. Puliani G, Chiefari A, Mormando M, et al. New insights in PRRT: lessons from 2021. Front Endocrinol (Lausanne) 2022; 13: 861434.
57. Fazio N, Kulke M, Rosbrook B, et al. Updated efficacy and safety outcomes for patients with well-differentiated pancreatic neuroendocrine tumors treated with sunitinib. Target Oncol 2021; 16: 27-35.
58. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl JMed v2011; 364: 514-523.
59. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016; 387: 968-977.
60. Das S, Al-Toubah T,Strosberg J. Chemotherapy in neuroendocrine tumors. Cancers (Basel) 2021; 13:4872.
61. Chan DL, Bergsland EK, Chan JA, et al. Temozolomide in grade 3 gastroenteropancreatic neuroendocrine neoplasms: a multicenter retrospective review. Oncologist 2021; 26: 950-955.
62. Ricci C, Partelli S, Landoni L, et al. Survival after active surveillance versus upfront surgery for incidental small pancreatic neuroendocrine tumours. Br J Surg 2022; 109: 733-738.
63. Fazio N. Watch and wait policy in advanced neuroendocrine tumors: what does it mean? World J Clin Oncol 2017; 8: 96-99.
64. Emery J. Cancer survivorship-the role of the GP. Aust Fam Physician 2014; 43: 521-525.
65. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncology 2017; 3: 1335-1342.