Secondary fracture prevention. Ensuring the first fracture is the last
Anyone who has suffered a minimal trauma fracture is at heightened risk of future fractures and the associated morbidity and mortality. Safe and effective pharmacological and nonpharmacological approaches are available to reduce the risk of recurrent fractures. It is critical that patients with incident fragility fractures are identified and managed early to make the first fracture the last.
- Sustaining a minimal trauma fracture (MTF) increases the risk of further fractures by at least twofold.
- Assessment of clinical risk factors for osteoporosis is mandatory in every patient who presents with MTF.
- Investigations include a bone mineral density scan and blood tests (e.g. vitamin D level).
- Secondary causes of osteoporosis should be considered in all patients.
- Safe and effective pharmacological and nonpharmacological treatments are available to prevent fractures; however, despite their wide availability a large treatment gap remains.
- Systematic, co-ordinated approaches to secondary fracture prevention have been shown to improve treatment initiation rates and prevent further fractures.
Osteoporosis is characterised by microarchitectural deterioration of bone and low bone mass leading to reduced bone strength, which predisposes to fractures due to minimal trauma (i.e. an impact equivalent to a fall from standing height or lower).1 Minimal trauma fractures (MTFs) are also referred to as fragility or osteoporotic fractures. In Australia, 140,822 MTFs were sustained in 2012; by 2022, this figure is expected to increase to 183,105.2 More than half of postmenopausal women and a third of men over the age of 60 years will experience at least one MTF during their remaining lifetime.3
MTF is associated with significant morbidity and mortality. Individuals who have sustained MTF are at least two times more likely to sustain further fractures, especially within the first two years after the initial fracture.4,5 People with minimal trauma vertebral or hip fractures have at least a four-times increased risk of refracture.6-8 Although men are less likely to sustain initial fractures than women, men are more likely to sustain a further fracture and have higher mortality after an initial fracture.5,9 Furthermore, excess mortality occurs after all proximal osteoporotic fractures, particularly in the first five years, and this risk may persist for up to 10 years after a hip fracture.9
Pharmacotherapy for osteoporosis is safe and effective in reducing the risk of further fracture by up to 70%. However, despite the wide availability of antiosteoporosis drugs, fewer than 30% of patients are appropriately assessed and treated for the underlying cause of their fragility fracture (i.e. osteoporosis).10 Two large retrospective studies of general practice in Australia revealed less than 33% of patients presenting with a fragility fracture received osteoporosis pharmacotherapy.11,12 This gap in treatment has also been demonstrated in hospitals.13 To address the care gap in osteoporosis management, centres across the world have developed systematic, co-ordinated interventions to identify patients who have sustained a minimal trauma fracture, investigate and determine fracture risk and initiate interventions to reduce fracture risk. A 2013 systematic review divided interventions into four models of care according to intervention intensity, from most intensive (Type A) to least intensive (Type D) (Table 1).14 The effectiveness of each model of care correlates with the intervention intensity, with the most intensive being most effective and least intensive being least effective.
Effective secondary fracture prevention programs (SFPPs; also known as fracture liaison services or FLSs) cover the three ‘i’s’ of fracture prevention: identification, investigation and initiation of therapy. These programs have been shown to improve osteoporosis treatment initiation and reduce refracture rates, compared with standard care.
Being outpatient services, most SFPPs preferentially capture patients with nonhip, nonvertebral fractures, whereas patients who are institutionalised, frail or have sustained hip fractures are often missed. Therefore, inpatient orthogeriatric services play a critical role in managing this group of patients. In addition, most SFPPs fail to capture patients with vertebral fractures, as these fractures are often asymptomatic or patients don’t present to hospital. Finally, most SFPPs are under-resourced and lack technology for the systematic identification of patients with fragility fractures and, as a result, only capture about 20% of patients who require care.
It is therefore important to widen the net and go beyond traditional hospital-based SFPPs. In this context, it is important to realise that primary care plays a critical, if not the most important and central role in identifying and managing patients with osteoporotic fractures. This article explores how an at-risk patient may be managed for fracture prevention through an in-depth case vignette. Supported by a large NHMRC grant, we are currently trialling a new model of care that integrates primary and community care with secondary and tertiary hospital services.
Risk factor assessment
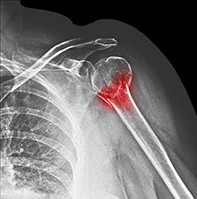
Norma is a 68-year-old woman who has sustained a left proximal humeral fracture after slipping on a wet floor at the local shopping centre. She has a history of asthma, hypertension and breast cancer, which is in remission. How would you assess the clinical risk factors for Norma’s MTF?
A clinical osteoporosis risk-factor assessment should be undertaken for every patient who sustains an MTF. This initially involves a detailed history and examination. Clinical risk factors for osteoporotic fracture are listed in the Box, but some of the more important are prior fragility fracture, recurrent falls, older age and female sex. Specific assessment should determine estimated height loss of more than 2.5 cm, long-term corticosteroid exposure, coeliac disease, malabsorptive symptoms, parental history of hip fracture and a history of hyperthyroidism. In women, the age of menarche and menopause, as well as a history of oligomenorrhoea, should be determined to elicit the degree of oestrogen exposure before the use of menopausal replacement therapy (MRT). In men, symptoms of hypogonadism, including loss of libido and decreased shaving frequency, should be ascertained. Enquiry regarding lifestyle factors including smoking, alcohol consumption, dietary calcium intake, sunlight exposure and exercise will be helpful to later tailor individual nonpharmacological therapy to each patient.
Targeted examination to find evidence suggestive of vertebral fractures includes checking for thoracic kyphosis, focal bony tenderness and an increased wall-to-occiput distance (greater than 0 cm). It is important to check for evidence of a secondary cause for osteoporosis such as thyrotoxicosis (e.g. tachycardia, tremor) or Cushing’s syndrome (e.g. hypertension, facial plethora, abdominal striae, proximal muscle weakness, bruising). In men, evidence of hypogonadism such as sparse facial and body hair and gynaecomastia should be elicited; testicular sizes should be recorded if there is evidence of hypogonadism. Recording the patient’s weight and height are simple measures to monitor; height loss of more than 2.5 cm may suggest further vertebral fractures, and weight loss may be associated with a reduction in bone mineral density (BMD). Gait should be assessed for ataxia or focal weakness, and postural blood pressure (BP) checked to exclude postural hypotension.
Investigations
On further enquiry, you establish that Norma’s breast cancer was oestrogen- and progesterone-receptor positive and managed with a lumpectomy and radiotherapy, followed by aromatase inhibitor therapy over the past three years (ongoing). She underwent menopause at 55 years of age and did not receive MHT. Her hypertension is well managed on a single agent and asthma well controlled on inhaled corticosteroids and a beta-agonist only. She has never required oral corticosteroids. Clinical examination reveals a BP of 130/80 mmHg with no postural drop. There is no evidence of Cushing’s syndrome and she is euthyroid. She has mild thoracic kyphosis but no focal tenderness. Wall-to-occiput distance is 2 cm. Her gait is normal with no focal neurological deficit. What further investigations would you perform?
Measurement of BMD is an important component of fracture-risk assessment that is best performed using a dual-energy x-ray absorptiometry (DXA) scan. A DXA scan is safe and noninvasive and involves low levels of radiation exposure. Thus, DXA should be used for initial assessment of BMD and long-term monitoring. A DXA scan may not be necessary in all patients to assess fracture risk. For example, a DXA scan may not significantly alter risk assessment or management decisions among frail elderly patients with hip or vertebral fractures. In addition, a person who has had MTF but has normal BMD on DXA can still have osteoporosis. Secondary causes of osteoporosis should be considered in all patients, particularly those with multiple fractures or a Z-score (the age- and sex-matched BMD measurement) below –2.
Clinicians should interpret the T-score results with caution as the lumbar spine T-score may be falsely elevated by osteoarthritis or aortic calcification. Under these circumstances, if further assessment of BMD is required, a quantitative CT may be considered if results from CT would change management or if the clinical picture is not in keeping with the DXA scan results. CT is, however, associated with radiation exposure more than 20 times higher than that from DXA and should therefore not be the first-line investigation to assess BMD and should not be used to monitor change. Notably, hip T-scores are not affected by osteoarthritis as the joint is outside of the scanned region.
Laboratory investigations for Norma include assessment of baseline creatinine levels (to guide treatment choice), serum calcium levels (to determine hypercalcaemia or hypocalcaemia), phosphate levels (to look for hypophosphataemia) and vitamin D and thyroid stimulating hormone levels (to look for hyperthyroidism). Other investigations to determine secondary causes of osteoporosis include a coeliac disease screen (including measuring tissue transglutaminase IgA antibody levels) as well as serum protein electrophoresis, immunoelectrophoresis and serum free light chains to detect myeloma. As more than 50% of patients with a vertebral fracture are asymptomatic, spine imaging should be performed using a thoracolumbar spine x-ray or vertebral fracture assessment to look for prevalent vertebral fractures, which will have significant implications for future fracture risk.
Management
Preventing further fractures
Norma’s DXA scan reveals a T-score of –2.0 at the lumbar spine and –2.1 at the femoral neck. Her pathology test results show normal renal function and normal levels of calcium, phosphate and thyroid stimulating hormone. Her 25-hydroxyvitamin D level is 30 nmol/L. Results of her screening tests for coeliac disease and myeloma are negative. Her spine x-ray reveals a T12 vertebral fracture with 25% anterior height loss (Figure). How would you reduce Norma’s risk of further fractures?
With a recent nonvertebral fracture, past vertebral fracture, osteopenia and current aromatase inhibitor use, Norma is at very high risk of further osteoporotic fracture and would benefit from specific osteoporosis pharmacotherapy. For patients who are at high risk of falls, implementing strategies to reduce the risk of falls is appropriate. Online absolute fracture risk calculators (e.g. Fracture Risk Assessment Tool [www.shef.ac.uk/FRAX] or Garvan Fracture Risk Calculator [www. garvan.org.au/bone-fracture-risk]) are useful tools to help determine an individual’s fracture risk and to guide therapy and patient education. A number of nonpharmacological and pharmacological approaches are available to reduce Norma’s risk of further fractures.
Nonpharmacological therapy
Nonpharmacological therapy should be used as an adjunct to pharmacological therapy and not a replacement. Australian guidelines recommend several nonpharmacological strategies for patients with osteoporosis including multifactorial falls prevention strategies, weightbearing exercises and optimising vitamin D and calcium intake. Multifactorial falls prevention includes exercises to improve muscle strength and balance, as well as reviewing environmental hazards, vision and risks from medications (e.g. antihypertensives, sedatives). A serum vitamin D level of above 50 nmol/L is appropriate (although a level above 70 nmol/L is preferable) and a dietary calcium intake of about 1300 mg daily should be maintained. Vitamin D levels can vary from 10 to 20 nmol/L between summer and winter and should be interpreted in the context of the season. Calcium supplementation is also controversial, with conflicting data regarding its effect on cardiovascular risk.15,16 Overall, if adequate dietary calcium intake cannot be maintained, then supplementation up to the recommended levels is appropriate.
Pharmacotherapy
Norma has osteoporosis with a recent MTF despite having a BMD in the ‘osteopenic’ range. Given that Norma would benefit from osteoporosis pharmacotherapy in addition to nonpharmacological measures, which pharmacological therapy would you initiate for secondary fracture prevention?
The selection of osteoporosis pharmacotherapy should be determined based on a patient’s comorbidities, likely side effects, patient compliance and patient preference. Medications for the treatment of osteoporosis, along with their antifracture efficacy and adverse effects, are shown in Table 2.
Bisphosphonates and denosumab
Antiresorptive agents in the form of bisphosphonates and denosumab are first-line agents for the treatment of men and postmenopausal women with osteoporosis. The antiresorptive medications are subsidised by the PBS for any patient with MTF, irrespective of BMD T-score, but it is up to the treating physician to balance the benefits and risks of starting therapy for each patient.
Oral bisphosphonates (alendronate, risedronate), intravenous bisphosphonates (zoledronic acid) and denosumab have similar antifracture efficacy (Table 2), but each has unique side effects that need to be considered before beginning therapy. Oral bisphosphonates can be associated with exacerbation of reflux symptoms and oesophagitis, and these side effects should be kept in mind on follow-up visits.17 Moreover, they require specific administration instructions (remaining upright for at least 30 minutes following ingestion) and, like many oral medications, have poor patient compliance after one year.18 However, compliance is safeguarded with yearly intravenous infusions of zoledronic acid or six-monthly subcutaneous injection of denosumab. The most common adverse effect of zoledronic acid is an influenza-like or acute phase reaction, which occurs in about 30% of patients after the first infusion, although its severity diminishes with subsequent infusions.19 Bisphosphonates should be avoided if creatinine clearance is less than 30 mL/min.
Denosumab has a slightly higher risk of causing eczema (3.0%) compared with placebo (1.7%) and, in the first three years of therapy, cellulitis but is otherwise well tolerated.20 Notably, there is no evidence of an increased risk of cellulitis over placebo beyond three years follow up.20 Although denosumab is not renally cleared (in contrast to bisphosphonates), it should be used with caution when creatinine clearance levels are below 30 mL/min because of an increased risk of hypocalcaemia. Moreover, as chronic kidney disease affects bone health in different ways, specialist input should be sought for patients with this disease.
Omitting or delaying a dose of denosumab results in rapid rebound bone loss over the next six to 12 months; there have been case reports of vertebral fractures occurring after denosumab cessation.21 It is therefore critical to adhere to its six-monthly regimen. Bisphosphonate cessation, on the other hand, is not associated with rebound fractures or rapid bone loss. Overall, denosumab and bisphosphonates are both excellent options for patients at high risk of osteoporotic fracture. To reduce the risk of hypocalcaemia with any of these agents, the patient’s vitamin D level should be above 50 nmol/L before starting therapy.
Other antiresorptive and anabolic agents
Selective oestrogen receptor modulators (SERMs), such as raloxifene, have oestrogen agonist activity on the bone and antagonist activity at the breast. Raloxifene has been shown to reduce the risk of vertebral fractures only and may be considered for younger postmenopausal women (aged in their 50s or early 60s) with primarily vertebral osteoporosis.22 However, raloxifene may result in hot flushes and increases venous thrombosis risk.
MRT may also be considered for younger postmenopausal women at moderate to high risk of fracture and with menopausal symptoms. A subanalysis of the Women’s Health Initiative study showed that adverse effects of MRT, including risk of stroke and breast cancer, increased only after 60 years of age.23 Thus, administering MRT up to 60 years of age or within 10 years of menopause may be an option for a woman aged in her 50s who has osteoporosis.
Teriparatide is the active fragment (the first 34 amino acids) of human parathyroid hormone and, until very recently, was the only bone building (‘bone anabolic’) agent available in Australia. It has been shown to reduce the risk of vertebral and, less so, nonvertebral fractures.24 Some data suggest it has also analgesic effects (reduced back pain).25 There is anecdotal evidence for the use of teriparatide in patients with severe osteonecrosis of the jaw.26 Teriparatide is contraindicated in patients at high risk of osteosarcoma, including those with Paget’s disease or bone metastases or who have had previous radiotherapy.
A second anabolic agent available in Australia since April 1, 2021 is romosozumab, a monoclonal antibody that inhibits sclerostin. Sclerostin suppresses osteoblast activity and thereby bone formation. By neutralising sclerostin, romosozumab promotes bone formation and decreases bone resorption. It has been shown to reduce the risk of vertebral and clinical fractures compared with both placebo and alendronate. In the ARCH trial, a randomised controlled trial comparing romosozumab with alendronate, 50 of 2040 patients (2.5%) had serious cardiovascular events in the romosozumab group versus 38 of 2014 patients (1.9%) in the alendronate group.27 A causal relationship between romosozumab and these events has not been established. However, romosozumab should not be initiated in those who have suffered from a myocardial infarct or stroke in the preceding year.
Both teriparatide and romosozumab are listed on the PBS for patients with severe osteoporosis (BMD T-score of −3.0 or lower plus two or more fractures, one of which must have occurred after 12 months of antiresorptive therapy) and requires initiation by a specialist.
Duration of pharmacotherapy
Norma commences annual zoledronic acid infusions, which she tolerates well. Three years later, at the age of 71 years, her BMD is stable and she has remained fracture-free. How long would you continue her bisphosphonate therapy?
Guidelines recommend continuing bisphosphonate therapy among patients who are at high risk of recurrent fracture, especially those with a T-score at the hip of –2.5 or lower at the time of reassessment or with previous hip or vertebral fractures, as is Norma’s case.28 Cessation of denosumab is not advised because of its rapid offset of action after six months of administration.
Although long-term use of bisphosphonates and denosumab may be associated with higher risk of atypical femoral fracture or osteonecrosis of the jaw (up to 100 cases per 100,000 person-years), this risk is much lower than the risk of recurrent osteoporotic fracture.29 Minimising the risk of osteonecrosis of the jaw, especially in those with other risk factors such as cytotoxic chemotherapy, involves patients maintaining good oral hygiene. They should be encouraged to undergo regular dental visits and inform their dentist that they are taking antiresorptive therapy for osteoporosis.
In making treatment decisions, the very low risk of these events must be weighed against the significant benefits of treatment in preventing osteoporotic fractures. Overall, there is no specific duration for which antiresorptive therapy should continue, but in those who are at very high risk of recurrent fracture, treatment may be continued for more than 10 years.
Conclusion
Sustaining an MTF signifies an increased risk of further fracture and increased mortality risk. Investigations including a full clinical risk-factor assessment with BMD scan (with the exception of frail elderly who have sustained a hip fracture) are mandatory in all patients who sustained an osteoporotic fracture to initiate effective treatment and long-term management. Treatment decisions should be guided by the risk of refracture, the patient’s comorbidities, likely compliance and preferences, and medication side effects. ET
COMPETING INTERESTS: None.